Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
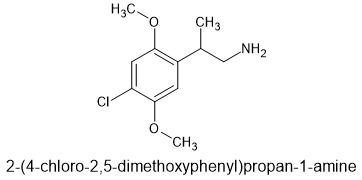
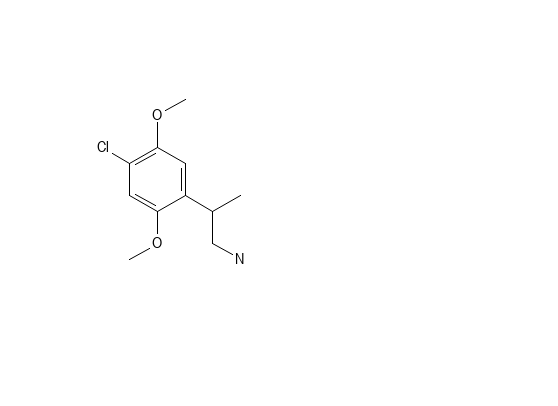
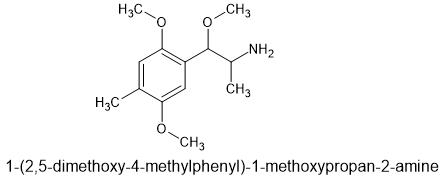
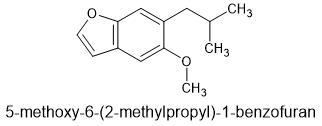
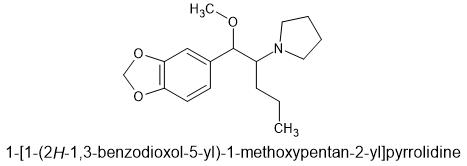
Of course, - MMDA-2 has the 2,5-domethoxy making it's 5HT2a affinity much higher. And the 4 is part of the MD ring. I don't know dosage, but suspect it's much lower. The MD ring is removed by the body so duration shouldn't be to long.
But COST.
I learnt that you had to retail at x5 production to cover all other costs and produce profit. I sense the precursor would make this too costly.
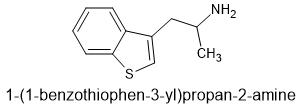
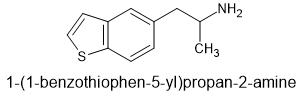
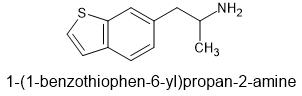
I guess someone could extent 6APB? THAT might actually be better as one methoxy (as part of ring) will be planer - key to 5HT2a affinity... why bromodragonly fly was so potent. those planer rings.
But COST.
I learnt that you had to retail at x5 production to cover all other costs and produce profit. I sense the precursor would make this too costly.
I guess someone could extent 6APB? THAT might actually be better as one methoxy (as part of ring) will be planer - key to 5HT2a affinity... why bromodragonly fly was so potent. those planer rings.