-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Post a chemical that you think has some serious potential to become a winner in the RC market
- Thread starter simstim
- Start date
Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
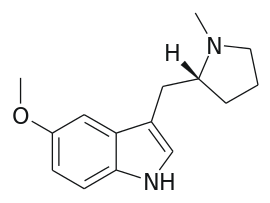
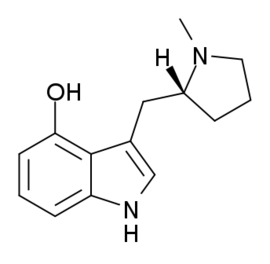
Now they ARE interesting. But how facile is their synthesis? IF you can find the appropriate amino-acid then if you feed it to the right mushrooms, it will add the 4-OH. In fact, is the 4-OH required?
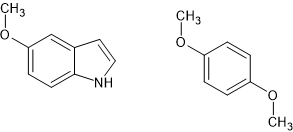
Do you see how the 2,5-dimethoxy and 5-methoxy insole overlay? With that in mind, maybe you can see how 7-methyl AMT overlays 3-methoxy-4-methyl amphetamine (which is MDA-like)?
And both the ring-substituted indole and the ring-substituted PEA are chiral with only one of the 2 isomers having 5HT2a affinity. So you can see how it's possible to make an indole that is purely an entactogen.

Do you see how the 2,5-dimethoxy and 5-methoxy insole overlay? With that in mind, maybe you can see how 7-methyl AMT overlays 3-methoxy-4-methyl amphetamine (which is MDA-like)?
And both the ring-substituted indole and the ring-substituted PEA are chiral with only one of the 2 isomers having 5HT2a affinity. So you can see how it's possible to make an indole that is purely an entactogen.
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
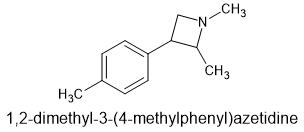
The non-2-methyl version is for surecovered in this patent. This may be covered as well
It is the 2-methyl-3-phenylazetitidine of MDA. I'm sure it's not that simple to prepare. This patent has everything from 3-phenylazetidine analogs of 2c-x, to ring substituted 3-phenylazetidine-NBOMe's and the 3-phenylazetidine-FLY series.
Here is what I want to call MDPA.
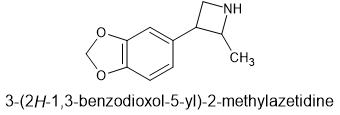
It is the 2-methyl-3-phenylazetitidine of MDA. I'm sure it's not that simple to prepare. This patent has everything from 3-phenylazetidine analogs of 2c-x, to ring substituted 3-phenylazetidine-NBOMe's and the 3-phenylazetidine-FLY series.
Here is what I want to call MDPA.

Last edited:
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
And here is the related patent.
WO2022221774 ARYL-RING-SUBSTITUTED 3-PHENYLAZETIDINES AND THEIR USE IN METHODS FOR TREATING 5-HT2 RESPONSIVE CONDITIONS
The invention features compounds and pharmaceutical compositions useful for treating 5-HT2 responsive conditions. Also provided are methods of using the compounds or compositions of the invention for treating 5-HT2 responsive conditions in a subject in need thereof.
patentscope.wipo.int
Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
BTW I think prodrugs are the way to go. Other have noted protecting groups that can be removed simply by boiling a solution of the compound in water.
That way, it's the end-user who is actually producing the active. Law enforcement might close down a reasonable fraction of large labs, but if everyone who buys a gram of the protected version, that means every user is in theory the 'producer'.
And every time a prodrug is banned, we just use another protecting group. I mean, you can place protecting groups onto heroin. So 'cooking' the stuff would make heroin.
I carefully avoid ever breaking the law. But it's actually impossible to ban hundreds of thousands of inactive compounds because it would impact research so hugely. If they simply made some safer things legal, likely nobody would bother with the illegal ones.
That way, it's the end-user who is actually producing the active. Law enforcement might close down a reasonable fraction of large labs, but if everyone who buys a gram of the protected version, that means every user is in theory the 'producer'.
And every time a prodrug is banned, we just use another protecting group. I mean, you can place protecting groups onto heroin. So 'cooking' the stuff would make heroin.
I carefully avoid ever breaking the law. But it's actually impossible to ban hundreds of thousands of inactive compounds because it would impact research so hugely. If they simply made some safer things legal, likely nobody would bother with the illegal ones.
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
βk-N-methyl-5-IT
Beta Keto n-methyl-5-IT
Why the beta ketone? Well it serves a functional purpose here because amphetamine SAR studies have shown beta ketones don't fit the MAO enzyme properly and 5-IT is an MAOi triple monoamine releasing agent.
For instance 4-methoxy-amphetamine and 4-methoxy-methamphetamine are both potent MAOis but their beta ketone derivatives are much weaker MAOis than plain amphetamine. Even the beta ketone of 4-methylthioamphetamine is relatively weak compared to the amphetamine (45uM for the ketone and .2uM for the amphetamine for MAO-A inhibition).
Why n-methyl? To prevent the formation of a dimer between the oxygen and the nitrogen.
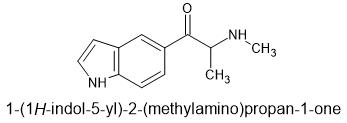
Entactogens of the future!!
Here is the relevant SAR study regarding substituted amphetamines as MAOis
 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
Beta Keto n-methyl-5-IT
Why the beta ketone? Well it serves a functional purpose here because amphetamine SAR studies have shown beta ketones don't fit the MAO enzyme properly and 5-IT is an MAOi triple monoamine releasing agent.
For instance 4-methoxy-amphetamine and 4-methoxy-methamphetamine are both potent MAOis but their beta ketone derivatives are much weaker MAOis than plain amphetamine. Even the beta ketone of 4-methylthioamphetamine is relatively weak compared to the amphetamine (45uM for the ketone and .2uM for the amphetamine for MAO-A inhibition).
Why n-methyl? To prevent the formation of a dimer between the oxygen and the nitrogen.

Entactogens of the future!!
Here is the relevant SAR study regarding substituted amphetamines as MAOis
Amphetamine Derivatives as Monoamine Oxidase Inhibitors - PMC
Amphetamine and its derivatives exhibit a wide range of pharmacological activities, including psychostimulant, hallucinogenic, entactogenic, anorectic, or antidepressant effects. The mechanisms of action underlying these effects are usually related ...
Last edited:
Delmonte421
Bluelighter
- Joined
- Oct 16, 2022
- Messages
- 1,117
I did 2cb once it was a fun mix between tripping and MDMA but not anything that I would say had any medical value. The structure is so closely related mescaline correct?If the beta ketone solves the MAOI issue, the moiety could be added to 2-C-T-2, 2-C-T-7 and so on. We got as far as bk-2CB when UK law stopped further work.
Here is the 2c Family for people interested in what makes 2C-p vs 2c-E etc.... 2c-p was very interesting cause its effects are logarithmic
Last edited:
Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
The ketone lowers the LogP so bk-2CB was only about ⅕ of the parent compound. It APPEARS to be quite non-toxic when a friend decided 'it hadn't worked' and took 240mg.
I suggest that while a stupid thing to do, the fact that their were no side-effects or signs of toxicity presage well for said modification. Maybe bk-2-C-T-7 or even bk-DOM would be more viable and reasonable options. My friend is a small guy and from his (somewhat disjointed) account, it would appear to be excreted via the kidneys. Of course, 1 person does not imply safety.
One would have to be most careful to test for hepatotoxicity but if it's no longer reliant on a key enzyme, that could make them less liable to interaction with other medicines.
I suggest that while a stupid thing to do, the fact that their were no side-effects or signs of toxicity presage well for said modification. Maybe bk-2-C-T-7 or even bk-DOM would be more viable and reasonable options. My friend is a small guy and from his (somewhat disjointed) account, it would appear to be excreted via the kidneys. Of course, 1 person does not imply safety.
One would have to be most careful to test for hepatotoxicity but if it's no longer reliant on a key enzyme, that could make them less liable to interaction with other medicines.
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
The problem with the beta keto 2C series is the formation of a dimer in water because they are primary amines. Shulgin said this and it's why people waited so long to attempt it.
The dimer formation may be the reason for the low oral potency as well.
The dimer formation may be the reason for the low oral potency as well.
SpiralusSancti
Bluelighter
I think it has a lot with individual metabolism. Friend who’s really experienced and hc with psychedelics, found bk-2c-b just as good as 2c-b and a bit less potent. It was too long ago for me to remember details but difference in doses between 2c-b and bk-2c-b get closer as dose gets higher for some reason, so at low doses 2c-b feels hugely more potent (as in x times more potent) than same amount of bk-2c-b but at really high doses it doesn't stay so (becomes % more potent, as in less than double for sure). Is that purely subjective experience, I don’t think so as I’ve seen both people saying bk version is garbage and other liking it just as much as “original”.
fastandbulbous
Bluelight Crew
The methoxyAPB compound is active. If it's anything like MMDA-2, it sounds really interesting, especially as having a carbon atom on ring opposite 2-aminopropyl chain is also rather like structure of DOM.Of course, - MMDA-2 has the 2,5-domethoxy making it's 5HT2a affinity much higher. And the 4 is part of the MD ring. I don't know dosage, but suspect it's much lower. The MD ring is removed by the body so duration shouldn't be to long.
But COST.
I learnt that you had to retail at x5 production to cover all other costs and produce profit. I sense the precursor would make this too costly.
I guess someone could extent 6APB? THAT might actually be better as one methoxy (as part of ring) will be planer - key to 5HT2a affinity... why bromodragonly fly was so potent. those planer rings.
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
I'm gonna go ahead and post this one here.
The beta ketone serves the purpose of disrupting function as an MAOi (N-Me-αMT is a putative triple monoamine releasing agent and an MAOi) and the n-methyl prevents the ready formation of a dimer.
βK-α,N-DMT aka beta keto n-methyl αMT
aka "tryptalone" as suggested as a name by @paracelsius in another thread
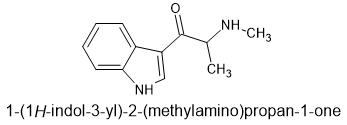
Wikipedia lists a 5-Fluro derivative of this and a relevant patent however I cannot retrieve the patent.
The beta ketone serves the purpose of disrupting function as an MAOi (N-Me-αMT is a putative triple monoamine releasing agent and an MAOi) and the n-methyl prevents the ready formation of a dimer.
βK-α,N-DMT aka beta keto n-methyl αMT
aka "tryptalone" as suggested as a name by @paracelsius in another thread

Wikipedia lists a 5-Fluro derivative of this and a relevant patent however I cannot retrieve the patent.
Last edited:
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
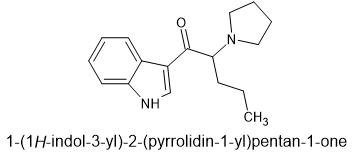
I wouldn't be surprised if this has already been prepared by someone once upon a time.
This was suggested by @paracelsius. I call it "tryptaPV". It is very similar to α-naphyrone which is relatively unknown pharmacologically according to wikipedia since all the research was done with β-naphyrone.
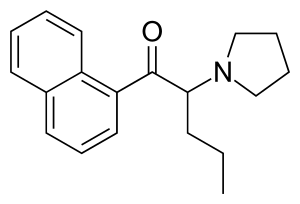
Here is α-naphyrone for comparison
This was suggested by @paracelsius. I call it "tryptaPV". It is very similar to α-naphyrone which is relatively unknown pharmacologically according to wikipedia since all the research was done with β-naphyrone.

Here is α-naphyrone for comparison

fastandbulbous
Bluelight Crew
I'm afraid that to work as a decent entactogen, there has to be an oxygen (or possibly other atom with lone pair of electrons) on the position meta orientated to the 2-aminopropyl chain. Attached to the phenyl ring, it is held pretty firmly, such that one of the lone pairs is in exactly the correct position to interact with the SERT. It's why 6-APB is infinitely better as an entactogen than 5-APB (or IAP).6-MeO-5-APIB
aka 6-methoxy-5(2-aminopropyl)isobenzofuran
The isobenzofuran of MMDA-2.
Should be purely psychedelic?
Hopefully the future will also bring...
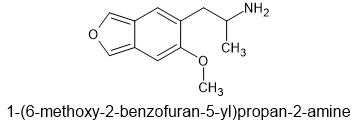
As regards the one I posted - 6-(2-aminopropyl)-5-methoxybenzofuran. I received a research paper that still hasn't been published, so I was asked to to send out any copies of the paper, as it has the university in question's name stamped on it, but it does refer to the meta orientated oxygen, specifically the positioning of the oxygen lone pair. As to activity, it tested positive with the rodent head twitch test, but no human has ever eaten any (as far as Iknow), so the finer entactogen/psychedelic aspects are still a mystery.
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
Well that could turn out to be a bit of a dud to use then. I wasn't too crazy about 5-APB. It was probably a commercial success for it's producers though.I'm afraid that to work as a decent entactogen, there has to be an oxygen (or possibly other atom with lone pair of electrons) on the position meta orientated to the 2-aminopropyl chain. Attached to the phenyl ring, it is held pretty firmly, such that one of the lone pairs is in exactly the correct position to interact with the SERT. It's why 6-APB is infinitely better as an entactogen than 5-APB (or IAP).
As regards the one I posted - 6-(2-aminopropyl)-5-methoxybenzofuran. I received a research paper that still hasn't been published, so I was asked to to send out any copies of the paper, as it has the university in question's name stamped on it, but it does refer to the meta orientated oxygen, specifically the positioning of the oxygen lone pair. As to activity, it tested positive with the rodent head twitch test, but no human has ever eaten any (as far as Iknow), so the finer entactogen/psychedelic aspects are still a mystery.
Edit: I would think that 5-APIB could be as good as IAP was.
Last edited: