-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Post a chemical that you think has some serious potential to become a winner in the RC market
- Thread starter simstim
- Start date
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
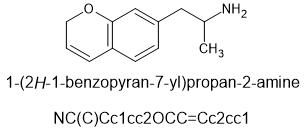
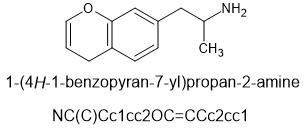
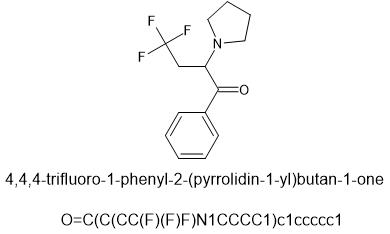
This chemical and it's n-methyl version are known. Whether they have been sampled by humans or not may be another story. I predict that they can be made available on the open market and will be useful.
@cosmic charlie this is the ring opened version of MDPM
β-MeO-MDA
Beta-methoxy-MDA
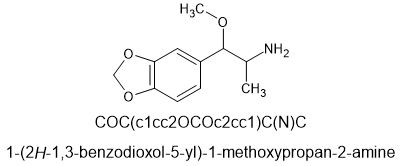
@cosmic charlie this is the ring opened version of MDPM
β-MeO-MDA
Beta-methoxy-MDA

AlsoTapered
Bluelighter
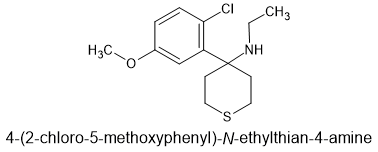
This is by no means the simplest nor the cheapest ketamine/methoxetamine analogue but I've put up the Roche patents on the 2-chloro-5-methoxy substitution being twice the potency of the m-methoxy (MXE) ring substitution and thus about seven times the potency of ketamine. The thianes are much more active than their cyclohexane counterparts and N-ethyl proves to give an appropriate balance of NMDA and dopamine activity. BUT given that the NMDA activity will be higher, maybe the N-methyl would be more appropriate. The N-isopropyl is also interesting (it was the most potent monosubstitution when PCP analogues were investigated) so that would also be worth looking at...
But it's a solid but novel design and given that K sells for £50/g, something that's x7 more potent would, I presume, fetch seven times the price IF it were sold as K. Goodness knows what one would choose to cut it with and I hate the idea of cutting a perfectly good drug but it seems that people prefer to pay £50/gram for a nice white powder than £350 or to pay £50 for 140mg. MAYBE £30 a point... but buyers seem to be at home with round numbers for round amounts.
Also, given the way things are going, their will be someone issuing a patent for α-Methyl sufentanil. From patent data, it would appear that the racemate is about x15 fentanyl i.e. x1200 M. BUT the key things are that it's TI is truly huge (>12000), it's duration is 4-6 hours, it's orally active and it's amenable to sustained release formulations (so one pill ever 12 or 24 hours) and to depot injections that would last for 28 days.
I also spent a few weeks looking at benzodiazepines. I found one that has a potency x10 that of flunitrazolam, a prodrug that would allow sustained release formulations (a pill every 12 or 24 hours) and once again, to depot injections that last 28 days. It's not some HUGE secret and most of it is known but nobody has put the ideas together, sadly/gladly.
If I had the money, I would patent the concepts and I feel certain that the drug research companies are JUST waking up to the fentanyl disaster and so in a year or two, they will wake up to the benzodiazepine disaster... but it will all be too late.
I COULD have samples made but that would be illegal and I don't 'do' illegal.
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
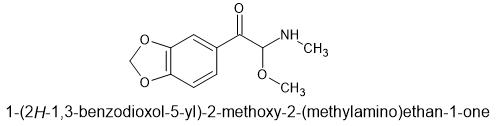
I have taken a look at this in 3d viewers and I believe that this is potentially at least as good as 4-MEC. Possibly this is the best mephedrone replacement since 4-MEC.
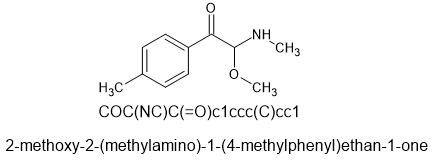
The non para-methyl compound is known and patented. I don't know what the patent is even about because it looks like it's in Chinese.
This is 2-methoxy-2-(methylamino)-1-(4-methylphenyl)ethan-1-one
aka
alpha-MeO-beta-keto-4-methyl-PEA
aka
α-MeO-β-keto-4-MePEA
SMILES=COC(NC)C(=O)c1ccc(C)cc1
The non para-methyl compound is known and patented. I don't know what the patent is even about because it looks like it's in Chinese.
This is 2-methoxy-2-(methylamino)-1-(4-methylphenyl)ethan-1-one
aka
alpha-MeO-beta-keto-4-methyl-PEA
aka
α-MeO-β-keto-4-MePEA
SMILES=COC(NC)C(=O)c1ccc(C)cc1

Last edited:
AlsoTapered
Bluelighter
How would you make it? 1 carbon with an O & N bonded to it?
So that's where you need to hit the organic chemistry books. EWGs reduce activity... but it's one for PubChem. It it simply does not exist, their is generally a practical reason/
So that's where you need to hit the organic chemistry books. EWGs reduce activity... but it's one for PubChem. It it simply does not exist, their is generally a practical reason/
AlsoTapered
Bluelighter
Forget the MD - find the PLAIN PEA.
AlsoTapered
Bluelighter
Well if the UNSUBSTITUTED version is too hard to make, how do you expect to make the ring-substituted versions?
Also ask HOW you would make ANY of these compounds. Yes, senior medicinal chemists merely have to find the papers and let post-grads do the heavy lifting.... but you don't end up as a senior medicinal chemist WITHOUT having proved you CAN make targets.
It took me 18 years of 12 hours a day, 6 days a week.
Also ask HOW you would make ANY of these compounds. Yes, senior medicinal chemists merely have to find the papers and let post-grads do the heavy lifting.... but you don't end up as a senior medicinal chemist WITHOUT having proved you CAN make targets.
It took me 18 years of 12 hours a day, 6 days a week.
Delmonte421
Bluelighter
- Joined
- Oct 16, 2022
- Messages
- 1,117
@simstim HIT THE BOOKS AND WATCH SOME VIDEOS That should cover orgo 1
here is orgo 2
I am unsure if @AlsoTapered was a TA during his graduate work but if you watch those videos, it'll help you understand more about the reactions and building the molecule. also I see your naming your compounds but im assuming that its not you but the program your using. The first thing you learn in orgo, is how to properly name compounds, IUPAC..... if your that interested you can always sit in on your local university organic classes, their usually huge lecture halls.
Once you understand the mechanic reactions etc then you can start looking at retro synthesis of your compounds and cost out your reagents and precursors.... knowledge IS POWER, remember...You wasted $150,000 on an education you coulda got for $1.50 in late fees at the public library
here is orgo 2
I am unsure if @AlsoTapered was a TA during his graduate work but if you watch those videos, it'll help you understand more about the reactions and building the molecule. also I see your naming your compounds but im assuming that its not you but the program your using. The first thing you learn in orgo, is how to properly name compounds, IUPAC..... if your that interested you can always sit in on your local university organic classes, their usually huge lecture halls.
Once you understand the mechanic reactions etc then you can start looking at retro synthesis of your compounds and cost out your reagents and precursors.... knowledge IS POWER, remember...You wasted $150,000 on an education you coulda got for $1.50 in late fees at the public library
simstim
Bluelighter
- Joined
- Apr 20, 2021
- Messages
- 6,606
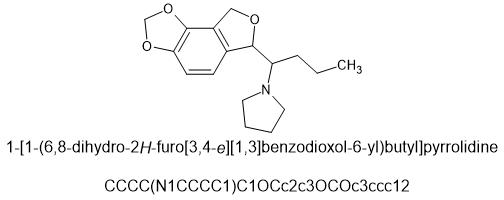
The idea behind this was to constrain the oxygen in mephedrone into a phthalane ring. This places the oxygen in roughly the same place as 4-MMC and the nitrogen looks to be in a very similar location in a 3d viewer. I don't know how to make this and would have to pay someone to do it.
SMILES=CNC(C)C1OCc2cc(C)ccc12
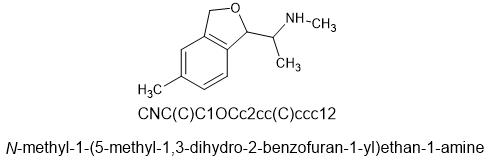
SMILES=CNC(C)C1OCc2cc(C)ccc12

Last edited:
AlsoTapered
Bluelighter
Well yes, rigid is good. But I do keep repeating 'aromatic relative to amine' and used amphetamine, cocaine and amfonelic acid as just SOME examples.
You also have to ask what the metabolic pathway will be.
Now, the methyl is GOOD. The body will oxidize the f**k out of that and it will be excreted as the salt of the carboxylic acid... but at best, you have too much bulk.
Don't forget, when one of the hydrogens of the methylenedioxy bridge of MDMA was replaced by an F, potency halved... And SERT receptors have the MOST space.
AR & 4MAR manage to be much like MDA without ring substitution (because they swap between 2 forms so it's like a mixture of the primary and secondary amine. Now p-halogen 4MARs were made and it was the p-F that was the MOST potent.... but I think that's because it's not easy for the body to metabolise. I've never touched it but some BLers who like stims LOVE it... it's x4.5 4MAR but duration is x3 longer....
So I suggest it would be LESS popular. We made p-Me AR, m-Me AR & then 3,4-MDAR, the last was x2 MDA in potency i.e. 50mg was like a strong pill....
And the intermediate amino-alcohols are not watched. Now I presume people go for the 4-M derivatives because KOCN cyclizes it (whereas with AR BrCN is used) BUT we found that making AR, the KOCN produced the substituted urea... and their are MANY simple dehydration reactions to drive it to the product.....
But that's all production.
You also have to ask what the metabolic pathway will be.
Now, the methyl is GOOD. The body will oxidize the f**k out of that and it will be excreted as the salt of the carboxylic acid... but at best, you have too much bulk.
Don't forget, when one of the hydrogens of the methylenedioxy bridge of MDMA was replaced by an F, potency halved... And SERT receptors have the MOST space.
AR & 4MAR manage to be much like MDA without ring substitution (because they swap between 2 forms so it's like a mixture of the primary and secondary amine. Now p-halogen 4MARs were made and it was the p-F that was the MOST potent.... but I think that's because it's not easy for the body to metabolise. I've never touched it but some BLers who like stims LOVE it... it's x4.5 4MAR but duration is x3 longer....
So I suggest it would be LESS popular. We made p-Me AR, m-Me AR & then 3,4-MDAR, the last was x2 MDA in potency i.e. 50mg was like a strong pill....
And the intermediate amino-alcohols are not watched. Now I presume people go for the 4-M derivatives because KOCN cyclizes it (whereas with AR BrCN is used) BUT we found that making AR, the KOCN produced the substituted urea... and their are MANY simple dehydration reactions to drive it to the product.....
But that's all production.
AlsoTapered
Bluelighter
The last is known and is active. that CF3 stops metabolism so duration goes up.... but it's mighty long as it is.
If someone making bath salts took your design, it would be legal and given increased LogP, more potent.
If someone making bath salts took your design, it would be legal and given increased LogP, more potent.