Delmonte421
Bluelighter
- Joined
- Oct 16, 2022
- Messages
- 1,117
rodent twitch test publication for info, I didn't know what he meantrodent head twitch test,
N&PD Moderators: Skorpio | someguyontheinternet
rodent twitch test publication for info, I didn't know what he meantrodent head twitch test,
do you have a background in medicinal chemistry and know about the metabolites of these compounds or do you have chemspider and just draw compounds that fallow the octane rule? Im being 100 seriousWell that could turn out to be a bit of a dud to use then. I wasn't too crazy about 5-APB. It was probably a commercial success for it's producers though.
No I don't know much too about the metabolism of these chemicals. I don't figure many of the chemicals that come out of china come out with known metabolisms in the beginning. or at least they didn't in the past. The point of the thread was to post chemicals that you believe have real potential to become a sales success on the research chemical market.do you have a background in medicinal chemistry and know about the metabolites of these compounds or do you have chemspider and just draw compounds that fallow the octane rule? Im being 100 serious
ok its of no offensive too you, but it let me know I can maybe explain some things a little further that might help you understand why or if these compounds can work. @Fertile has an extensive background in the structure of opiates and etc I am unsure of his knowledge with other novel compounds that relate to MDMA or meth but im sure he's on of the more knowledge people on this form.No I don't know much too about the metabolism of these chemicals. I don't figure many of the chemicals that come out of china come out with known metabolisms in the beginning. or at least they didn't in the past. The point of the thread was to post chemicals that you believe have real potential to become a sales success on the research chemical market.
It's not "post a new safe alternative to something that's probably illegal".
More like post something cheap yet effective that can be sold on the RC market for awhile.
I'm gonna go ahead and post this one here.
The beta ketone serves the purpose of disrupting function as an MAOi (N-Me-αMT is a putative triple monoamine releasing agent and an MAOi) and the n-methyl prevents the ready formation of a dimer.
βK-α,N-DMT aka beta keto n-methyl αMT
aka "tryptalone" as suggested as a name by @paracelsius in another thread
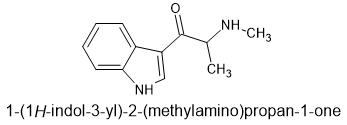
Wikipedia lists a 5-Fluro derivative of this and a relevant patent however I cannot retrieve the patent.
The alfa-methyl-bK-tryptamine is very interesting: It is a actually a selective Dopamine-Serotonin releaser with pretty zero Norpeniphrine. so that one would be pure MDMA-like entactogen about 2x as potent. Since no NE I suspect would be non-stimulant, no cardiovascular issues. Really very interesting compound: It is extremely rare (very rare) to have this kind of profile: Selective DA-SERT with no NE. very few compounds I come across are like that. Usually anything that release Dopamine would also release NE (very hard to separate the two like with this compound.I wouldn't be surprised if this has already been prepared by someone once upon a time.
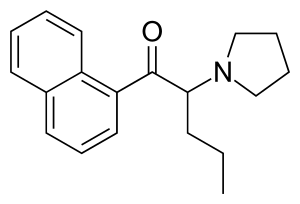
This was suggested by @paracelsius. I call it "tryptaPV". It is very similar to α-naphyrone which is relatively unknown pharmacologically according to wikipedia since all the research was done with β-naphyrone.
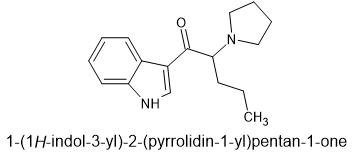
Here is α-naphyrone for comparison
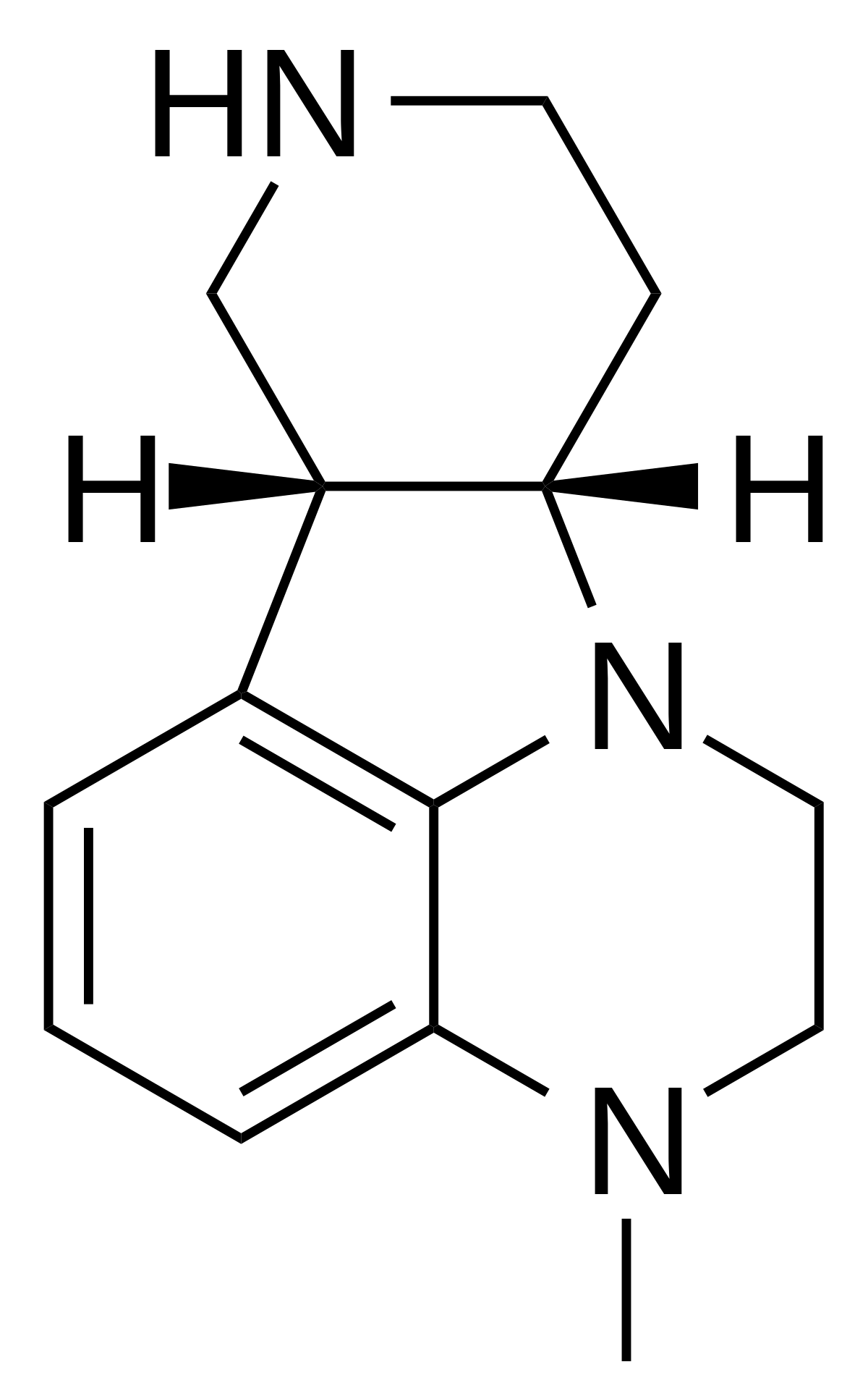

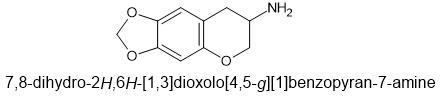
I have read you can synth codeine into hydromorphone with a 66% yield. So 300 mg codeine = 200 dilaudid. Only one ingredient needed if I remembr right.I'd say they belong to no one and/or all of us.
But I guess I understand your sentiment.
Are you saying that guy was lying about synthing an etorphine-level opioid from bupe? I mean he could have been, which is why I came to ask people more knowledgeable than me about chemistry topics.
It's amazing to think bupe could hold the potential to become a full agonist with some steps.
I was just curious is all. And enjoy learning, so no need for hostility.
I've read that mixing acetic anhydrous with almost any opiate/opioid changes it into another obscure opioid. Is this true/possible? And would acetic acid in vinegar be sufficient or are we talking needing lab grade equipment and chemicals?
Sorry for my ignorance.
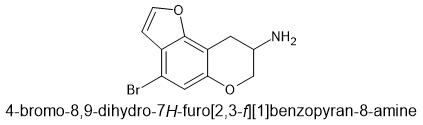
I did not say that it would be the most potent. Only predicting activity.The tetrahydropyran isn't planer so it will distort the position of the amine. You need to keep the oxygens planer to the benzene. Overlay it with TCB-2 to see if it's still viable... although whatever the case, I would wonder about metabolism. The amine might be oxidized and who knows what the activity of the metabolite will be.
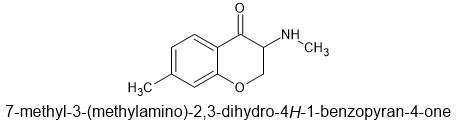
This molecules absolute mess of stereochemistry should not be seen as something to deter people from further researching such a promising compound.It has 8 enantiomers and so unless you intend to resolve and throw away 87.5%, it isn't as potent as one might imagine.
Carfentanil is achiral. So it's practially more potent. R-30490 is actually a lot more potent and is also achiral.
But moving to the Bentley compounds.... some of those derivatives are even more potent AND have much longer durations of action...
And their are worse....
This molecules absolute mess of stereochemistry should not be seen as something to deter people from further researching such a promising compound.
Just because we have more potent opioids doesn't mean we should stop researching new ones. If researchers had that mindset we would be stuck with our extremely primitive pharmacology of the past. Should we completely stop researching phytocannabinoids, drop to our knees and just accept that it doesn't get any better than something like THCP? Of course not, that's ridiculous.So you have investigated the resolution? Maybe 2 isomers is worth it - 8, NEVER. Especially since I listed MORE potent compounds that are achiral. I mean, R-30490 is more potent that the MOST potent OHMEfentanyl analogue.
Get a grip...
Is Sci-hub blocked where you are?MDAT fully substitutes for MDMA is rodents(https://pubs.acs.org/doi/abs/10.1021/jm00164a037). Also, there is this article about rigid analogs of DOM but I do not believe the article states that DOM-AT (the aminotetralin of DOM) substitutes for DOM. I do not have full access to the article, though. Does anyone know how to get access?

Potential psychotomimetics. 2. Rigid analogs of 2,5-dimethoxy-4-methylphenylisopropylamine (DOM, STP) - PubMed
Potential psychotomimetics. 2. Rigid analogs of 2,5-dimethoxy-4-methylphenylisopropylamine (DOM, STP)pubmed.ncbi.nlm.nih.gov