Nervewing
Bluelighter
- Joined
- Jan 5, 2016
- Messages
- 243
Oh yay I get to start one of these myself finally
An interesting arylcyclohexylamine that is purported to be hitting the markets soon
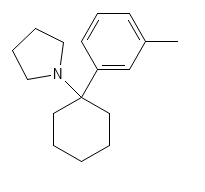
PCPy analogues haven't really been seen on the online rc market- 3-MeO-PCPy existed for a bit long ago but not too much information on it came out. Otherwise, PCPy has mostly just seen use as a street drug, where clandestine labs produce it to dodge around laws restricting PCP. As PCPy never really entered internet psychonaut circles (and in most cases was probably just being sold as PCP on the street), there is very little reporting on what its effects are. Shulgin however reports that it has remarkable sedative effects.
FWIW 3-MeO-PCPy is reported active in the range of 5-10 mg orally, it was described as being particularly stimulating in effect, an interesting contrast to what the base compound is purported to be like.
If we look at 3-MeO-PCP and compare it to 3-Me-PCP, we can infer that a similar pattern of effects may emerge (tho this is pure conjecture)- With the example of PCP analogues, this exchange in substitution sees a similar effect profile maintained and similar potency/bioavailability. I would feel safe in guessing that 3-Me-PCPy is also active in the 5-10 mg range, but there can always be aberrations in these patterns, anyone working with this should start from VERY low, like sub mg range until more information comes out.
The most detailed discussion and conjecture on this compound so far comes courtesy of Dr. Jason Wallach, from one of his chapters in the book "New Psychoactive Substances" (Maurer, Brandt, 2019)
"
Notably, 3-Me-PCPy showed high affinities for DAT, NET, and SERT compara-
ble to or higher than its NMDAR affinity (39, 45, 5.6, and 52.3 nM, respectively),
which was not shared by its PCP counterpart 3-Me-PCP. Furthermore, 3-Me-PCPy
was also shown to act as a reuptake inhibitor at these transporters (Wallach 2014).
3,4-MD-PCPy lacked significant affinity at DAT (IC50 > 10,000 nM) but showed
modest affinity at NET and SERT (Ki 1⁄4 420 and 115 nM). This is a similar trend to
that seen with 3-MeO-PCPy. The substituted PCPy series generally were found to
have higher affinities at monoamine reuptake transporters than their PCP
counterparts (Wallach 2014). Interestingly, some users have reported 3-Me-PCPy
to show a notable psychostimulant activity in addition to dissociative effects, which
suggests that this might be an unusual feature compared to structurally related
compounds. Interestingly, PCPy has been said to have sedative effects similar to
barbiturates (Shulgin and MacLean 1976). Four and eight mg doses (nasal insuffla-
tion of HCl salt) were reported to induce a relaxing ethanol-like state where sleep
was possible (a state uncommon with most other arylcyclohexylamines which tend
to be slightly stimulating especially at lower to medium doses) although it was
reported to clearly represent a dissociative effect and distinct in nature from the
effects induced by classic GABAergics including benzodiazepines and barbiturates
(personal communication). Efforts should be made to further investigate the poten-
tial pharmacological reasons for these apparently unique effects. For comparison
purposes, the IC50 values obtained from NMDAR binding studies of PCPy were
290 nM ([3H]-MK-801, rat cortex homogenate) (Stefek et al. 1990), 140 nM ([3
H] PCP, rat brain homogenate) (Kozlowski et al. 1986), and 200 nM ([3H]PCP, rat brain
homogenate) (Zukin and Zukin 1979). Displacement studies of [3H]PCP-specific
binding in rat olfactory bulb slices revealed PCPy (IC50 1⁄4 65 nM) to be more potent
than PCP (IC50 1⁄4 90 nM) and almost equipotent to TCP (IC50 1⁄4 54 nM). PCE was
shown to be more potent (IC50 1⁄4 15 nM), whereas ketamine was much less potent
(IC50 1⁄4 800 nM) (Quirion et al. 1981). When employing [3H]PCP-specific binding
to guinea-pig ileum preparations, the rank order of potency among a number of PCP
analogs showed (IC50): TCP (300 nM) > PCE (400 nM) > PCPy (450 nM) > PCP
(500 nM) > PCMo (1,000 nM) > ketamine (5,500 nM) (Gintzler et al. 1982).
However, it is unclear which receptor(s) were being labeled in this experiment,
which makes interpretation of these particular results challenging."
Anyways, figured I would dump all existing info so far, super excited to see what results come out of this one !
An interesting arylcyclohexylamine that is purported to be hitting the markets soon

PCPy analogues haven't really been seen on the online rc market- 3-MeO-PCPy existed for a bit long ago but not too much information on it came out. Otherwise, PCPy has mostly just seen use as a street drug, where clandestine labs produce it to dodge around laws restricting PCP. As PCPy never really entered internet psychonaut circles (and in most cases was probably just being sold as PCP on the street), there is very little reporting on what its effects are. Shulgin however reports that it has remarkable sedative effects.
FWIW 3-MeO-PCPy is reported active in the range of 5-10 mg orally, it was described as being particularly stimulating in effect, an interesting contrast to what the base compound is purported to be like.
If we look at 3-MeO-PCP and compare it to 3-Me-PCP, we can infer that a similar pattern of effects may emerge (tho this is pure conjecture)- With the example of PCP analogues, this exchange in substitution sees a similar effect profile maintained and similar potency/bioavailability. I would feel safe in guessing that 3-Me-PCPy is also active in the 5-10 mg range, but there can always be aberrations in these patterns, anyone working with this should start from VERY low, like sub mg range until more information comes out.
The most detailed discussion and conjecture on this compound so far comes courtesy of Dr. Jason Wallach, from one of his chapters in the book "New Psychoactive Substances" (Maurer, Brandt, 2019)
"
Notably, 3-Me-PCPy showed high affinities for DAT, NET, and SERT compara-
ble to or higher than its NMDAR affinity (39, 45, 5.6, and 52.3 nM, respectively),
which was not shared by its PCP counterpart 3-Me-PCP. Furthermore, 3-Me-PCPy
was also shown to act as a reuptake inhibitor at these transporters (Wallach 2014).
3,4-MD-PCPy lacked significant affinity at DAT (IC50 > 10,000 nM) but showed
modest affinity at NET and SERT (Ki 1⁄4 420 and 115 nM). This is a similar trend to
that seen with 3-MeO-PCPy. The substituted PCPy series generally were found to
have higher affinities at monoamine reuptake transporters than their PCP
counterparts (Wallach 2014). Interestingly, some users have reported 3-Me-PCPy
to show a notable psychostimulant activity in addition to dissociative effects, which
suggests that this might be an unusual feature compared to structurally related
compounds. Interestingly, PCPy has been said to have sedative effects similar to
barbiturates (Shulgin and MacLean 1976). Four and eight mg doses (nasal insuffla-
tion of HCl salt) were reported to induce a relaxing ethanol-like state where sleep
was possible (a state uncommon with most other arylcyclohexylamines which tend
to be slightly stimulating especially at lower to medium doses) although it was
reported to clearly represent a dissociative effect and distinct in nature from the
effects induced by classic GABAergics including benzodiazepines and barbiturates
(personal communication). Efforts should be made to further investigate the poten-
tial pharmacological reasons for these apparently unique effects. For comparison
purposes, the IC50 values obtained from NMDAR binding studies of PCPy were
290 nM ([3H]-MK-801, rat cortex homogenate) (Stefek et al. 1990), 140 nM ([3
H] PCP, rat brain homogenate) (Kozlowski et al. 1986), and 200 nM ([3H]PCP, rat brain
homogenate) (Zukin and Zukin 1979). Displacement studies of [3H]PCP-specific
binding in rat olfactory bulb slices revealed PCPy (IC50 1⁄4 65 nM) to be more potent
than PCP (IC50 1⁄4 90 nM) and almost equipotent to TCP (IC50 1⁄4 54 nM). PCE was
shown to be more potent (IC50 1⁄4 15 nM), whereas ketamine was much less potent
(IC50 1⁄4 800 nM) (Quirion et al. 1981). When employing [3H]PCP-specific binding
to guinea-pig ileum preparations, the rank order of potency among a number of PCP
analogs showed (IC50): TCP (300 nM) > PCE (400 nM) > PCPy (450 nM) > PCP
(500 nM) > PCMo (1,000 nM) > ketamine (5,500 nM) (Gintzler et al. 1982).
However, it is unclear which receptor(s) were being labeled in this experiment,
which makes interpretation of these particular results challenging."
Anyways, figured I would dump all existing info so far, super excited to see what results come out of this one !