-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
🌟🌟 Social 🌟🌟 Rectify's molecular poetry thread
- Thread starter Dresden
- Start date
Deleted member 81238
Bluelighter
I'm not worthy. 
I Think This Would Work.

Plus Br2,

No se requiere ephedrina.
Why not simply obtain L-PAC which is uncontrolled? - you can halogenate that quite simply.
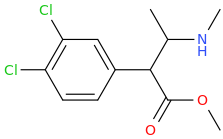
I think a great challenge would be to find a way to produce 4-MA$ from L-PAC in a facile manner. I've spent ages searching with no luck, but it's likely MY limitation rather than a limitation of the chemistry.
Last edited:
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 509
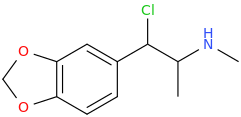
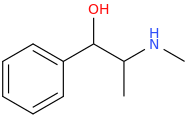
If I were you I would be tempted to contact a university and apply for it on your research thesis. Either that or the fluoxetine analogs made from ephedrine. You said Bounce has been made already though and you have tried it? Another leaving group that could be considered are tosylate or triflate groups.This Is Simpler Starting With Ephedrine:
Step 1: PBr3

Step 2: KCN, OH-
Step 3: Cl-TMS (chloro trimethyl silane)
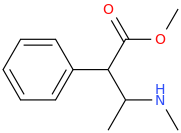
BOUNCE in ONLY 3 STEPS!
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,921
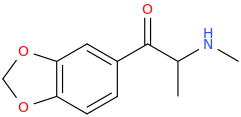
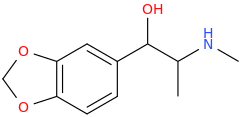
Yes, I tried some Bounce. Part stimulant, part opioid, part dissociative. Mainly stimulant. More potent than methamphetamine in terms of effects and requires a lower dosage. Is a "Dance Drug," like MDMA used to be when I could still get it. I can only, at this point, imagine what Superstar would feel like and whether the MDO-phenyl group can withstand Cl-TMS. You wouldn't happen to know, would you?
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 509
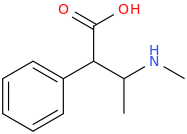
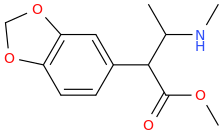
It's just an open chain analogue of ritalin though.
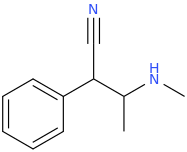
Well Bounce & Fluoxedrine are the only 2 of your ideas that are worth trying IMO. The rest can be discarded.
TRIZAK
1-phenyl-1-(4-trifluoromethylphenoxy)-2-methylaminopropane
This Is Simpler Starting With Ephedrine:
Yeah, it's MUCH easier staring from a controlled compound!
Did you even check what L-PAC was?
L-PAC is an ephedrine precursor, no?
And a methamphetamine precursor. It's a 1-pot reaction.