Original studies: https://www.shroomery.org/forums/showflat.php/Number/27850299/fpart/1/vc/1
I've had numerous pm's to condense the tek onto 1 page with pics, as lots of people are wanting to try this, so here it is...print this out and carry it with you and study it, it's super easy and fast I promise.
COMPLETE TEK ON HOW TO MAKE LSH and LSI combo or just LSI (your preference) on this one page with supporting studies, very simple:
Self trip with 600 seeds:
This trip report from Kash (long time LSA master) below is taken from post #3 of the beginning of this thread....keep in mind that LEMON JUICE also contains isovaleraldehyde, my best trip report using just a few HBWR was when I mixed them with fresh lemon juice from the fridge for a while, then consumed.
Do not use the cheap grocery store peppermint alcohol extract, instead buy real Peppermint oil max dosage: Adults: 0.2 to 0.4 mL (4 drops to 8 drops max for adults, DO NOT EXCEED: https://www.aafp.org/pubs/afp/issues/2007/0401/p1027.html
I still don't recommend using HBWR but it will still work to form LSI, see post #2 at very beginning of this thread: all about why this is so (MG vs HBWR seeds)...the main reason is that HBWR contains high amounts of ergometrine which causes cramping and vasoconstriction...whereas MG only has traces of it, I've used MG up to 25g and still no cramping or vasoconstriction. You can get way higher on MG seed LSI whereas with HBWR you will start to experience the somatic side effects of cramping way sooner due to the ergometrine if you are not careful on dosage, it's a balancing act with HBWR.
Psychonauts (psychedelic astronauts): you can still find isovaleraldehyde out there, you just have to look, try looking for the 25ml bottle.
Kash:
vespiary:
Hermes (lycaeum March 2003) on morning glory visuals & strength, extracted into cold distilled water with a squirt of lemon juice:
Nogal (the Nook):
dmthead420:

Easy steps, I've used this process over two dozen times in a 2 year period, it works with zero nausea:
---> You can even drink this LSH and LSI enhanced liquid extract at the same time you drop your LSD for an experience that is way beyond LSD, the LSH and LSI adds infinite dimensions to the normal LSD experience, like incredible (I mean phenomenal !) music enhancement, every sound in the music is "epic", infinite beauty enhancement, strong sensual feelings, strong neon like color saturation, flowing visuals, energy fields seen in constant motion around all objects, heavy tracers, etc. These qualities LSH and LSI adds are very similar to what cactus tea adds in my experience when added to LSD.
This is most likely due to LSH and LSI hitting 5 more adrenal recpetors associated with beauty, aesthetics and sensuality beyond normal LSD, see radioligand chart for LSH. I used this potion every time I tripped, you can even add it to cactus tea by taking drinking the LSH potion 1 hour later (always stagger 1 hour so the amides do not clash with the trace maoi's in cactus, only take in that order) it's incredible.
------------------------------------------------------------------------------
--> How to make the ZERO NAUSEA morning glory wine which is used for all aldehyde experiments, example: forming LSH and LSI from the LSA in the seeds: <--
How this works, from 2022 aldehydes paper, see 1960's study by Arcamone, go to post #1 to read all the studies (5 papers). LSH and LSI do make it to the bloodstream and urine, so it does indeed reach the brain so long as you keep it in it's original state (in acidic wine so it does not decompose back to LSA which will happen in plain water or nonacidic solutions) store in fridge or freeze in freezer to keep forever, drink as so.
Pic 1: Sherry wine is the wine highest in acetaldehyde.
Pic 2: With only a lamp on in garage, no overhead bright light: grind 15 grams or 525 heavenly blue morning glory seeds in a coffee grinder (35 seeds per gram) using 10 second grind with occasional shaking of coffee grinder, pause 5 seconds, then grind again 10 seconds, repeat x 4 times, you will end up with a dust like consisteny, all the alkaloids are extracted from within the tough rubbery embryo of the seed. 525 seeds x .01mg LSA per seed = 5mg LSA
Pic 3: Place ground seed dust into a 1/2 pint tall jar (*almart case of 12, canning section), add 4 shots of cold just opened fresh sherry wine from the fridge, and around 30mg of DL tartaric acid which aids the extraction (from hi media store, *mazon, auction or similar). Do not use plain L-tartaric acid used in wine making, get the good stuff, which contains the D isomer. D-lyergic acid amide isomer salts are the potent form of salts.
And also ADD a tiny splash or 1 to 2 teaspoons of acetic acid or VINEGAR (I use apple cider vinegar) to catalyze the condensations of the aldehyde to LSA: acetaldehyde in this case onto the amide of LSA, and isovaleraldehyde to form LSI. The vinegar acts as an important catalyst as it contains acetic acid, and speeds the formation of new adduct product, see study below:
From Mehra, R.K & Panya, K.C. 1938, The condensation of aldehydes with amides - part III, the condensation of cinnamaldehyde with acetamide. Proc Natl Acad Sci India Phys Sci 7(6). 376-380
NOTE: from paper: Acetamide (very similar to LSA) when cinnamaldehyde adduct added, forms: N,N'cinnamylidenediacetamide just as LSA + isovaleraldehyde forms LSI or Lysergic Acid Isovaleraldemide. RATS RESPOND TO it as if they had been given LSD, and this human rat loves the effects, the closest thing in nature I've ever experienced that is very similar to LSD. LOVE, LOVE, LOVE !!!



Cinnamaldehyde should not be used as it contains nearly twice the number of carbons and hydrogens and thus weak activity due to this (weights way too much)...only use isovaleraldehyde (weight is perfect) to form the potent LSI. However, I am showing the study so that you see an even "longer chain aldehyde than isovalearaldehyde" will even adduct onto acetamide or LSA as proven in study.
YOU WILL LOVE LSI, it is so very similar to LSD !!!



Re-seal your sherry wine by spraying wine preservation canister which has inert gas (contains argon, carbon dioxide, etc.) around ten dollars from *mazon into your wine before sealing with cork and placing back in fridge, this way the precious acetaldehyde in the wine will not oxidize to vinegar as normally happens over a 5 day period when corked without preserving.
Put lid on and shake contents for 1 to 2 minutes.
Place jar into fridge for 10 minutes after the 2 minute shaking, during this time the nauseating seed debris can be seen falling to the bottom.
Pic 4: Filter entire contents of jar thru a "coffee wire filter" from *almart or *amazon or your local grocery store sitting atop a glass, the liquid above the seed debris will filter real fast in seconds, then filter the debris at the bottom from the jar, once the debris is in basket, use a spoon to press down on it to get all the liquid out, this only takes around 10 seconds.
Pic 5: Place this filtered liquid in the fridge for 3 hours or even overnight, within 3 hours, all of the nauseating to the intestines seed debris will have fallen to the bottom. This process begins within an hour after sitting in fridge, check each hour and watch as the seed debris falls by around an inch each hour.
Pic 6: After 3 hours or longer, decant the liquid from above the bottom 1/4" of seed debris, this is what you want to consume, it is psychedelic and results in zero nausea, as there is no seed debris in it. DO NOT DRINK the SEED DEBRIS at very bottom or you will become nauseated and sick, don't worry, there are no alkaloids in it.
Note: never try to extract your seeds using just plain acidified water, I have tried this before, your seed debris will not separate out in the fridge, and you end up with a complete emulsion in the coffee wire filter which will not filter at all, wine is needed to effect the proper extraction, and allows the liquid to separate from the seed emulsion below, this will not happen with a plane water extration. This is how the ancient Aztec and Mayan extracted the seeds using balche, an alcohol they made themselves, see 2nd pic.
Rest of the pics:
sources of isovaleraldehyde: pure chemical (on line 25ml bottle) or you can use fresh lemon juice or pure peppermint oil, I prefer to use the pure chemical.
Use ice bath on your stirrer to keep wine as a combo of LSH + LSI (as LSH adduct acetaldehyde boils off at room temp, so always keep cold, to form LSI only, omit the ice bath, as LSI does not boil off till 198 degree F).
All the alkaloids are concentrated in the fridge decanted liquid above the seed debris. This contains your LSA which has adducted to the acetaldehyde in the wine, forming new amounts of LSH, since the wine is at ph=4, the new LSH adduct product will remain stable indefinately, store in fridge if you plan to use within a few days, or you can freeze and de-thaw in fridge overnight to use next day. It dethaws fast since it is wine.
You just formed LSH using the method above, to form LSI, simply take your 4 shot morning glory wine, and at this point add your 3 drops of isovaleraldehyde along with 1 to 2 teaspoons apple cider vinegar (if you don't have the pure chemical then add teaspoon or more of lemon juice, or several drops of pure peppermint oil) to form new molecule similar to LSD in every way. Be sure to spin for around 1 hour at high speed. I always use the pure chemical as I have it. Don't forget to add the apple cider vinegar as it contains the acetic acid catalyst which speeds up the adduct chemical reaction.









-----------------------------------------------------------------------------------------------------
References: Lysergic acid Isovaleraldemide, LSI
The aldehyde in peppermint oil and leaf and lemon juice is Isovaleraldehyde otherwise known as 3-methylbutanal. If you are a business or researcher you can order it directly, there are also two places that sell direct to individuals, just google it. They delivered it straight to my door, one place starts with a "*******" All you need to add is two drops of this to your LSA sherry wine liquid extract as it spins on the stir mantel for 1 hour at high speed, also must add 1 teaspoon of either vinegar or apple cider vinegar (my preference), as the acetic acid in the vinegar is the catalyst for the condensation of the isovaleraldehyde onto the LSA forming Lysergic Acid Isovaleraldemide, see study above.
There is .01mg LSA in 100 seeds (.01 x 100 = 1mg LSA), just a 100 seed extract has effects very similar to 100ug of LSD, LSI is 1/10th the potency of LSD. Immense visual power with closed eye geometrics, wild colors, music sounds bad ass, divine healing power. I have prepared an extract with 200 seeds and the strength increases similar to 200ug of acid.
I have been tripping my ass off every 14 days or so taking this LSI liquid extract around 1 hour after a very small bridgesii tea prepared with only 450 grams of bridgesii cactus chuncks from around the core (around 225mg mescaline), as I love the combo of cactus + LSD, this is no different. Yes, I have tried LSI many times without the cactus, super potent, highly recommend. I will be using LSI for the rest of my life.
This aldehyde is completely safe and has been administered in high does to rats with no ill effects. This aldehyde as you can see from paper makes up a substantial part of peppermint oil and leaf.
More on LSI:
chemical formula for entry #16....3-aminopentane = CH(C2 H5)2
chemical formula for isovaleraldehyde is (CH3)2 CH2 CH2 CHO
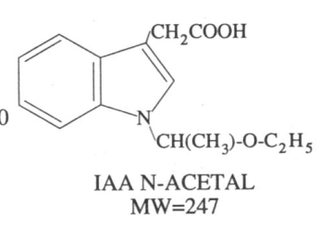
Notice isovaleraldehyde even LOOKS EARILY similar to the tale end of psilocin or DMT with nearly the same exact chemical formula as the tail end of psilocin or DMT, see pics in middle

diethylamine from LSD has 4 carbon groups and 11 hydrogen groups with a molecular weight of 73 g/mol.
Both the 3-aminopentane and the isovaleraldehyde of this new discovery have 5 carbon groups and 11 hydrogen groups (once the aldehyde attaches to the H already at the amide of LSA) and BOTH have exact same molecular weights of 87 g/mol similar to diethylamine molecular weight of LSD at 73g/mol.
The 3-aminopentane from Dr. Nichols and isovaleraldehyde discovery join at the R1 substitute of LSA with R=H still. This new aldehyde has same molecular weight as 3-aminopentane on the R1 substitute of LSA with R=H, see Dr. Nichol's paper on page 84, chart shown. This is one of only 2 entries in which the rats responded to it (3-aminopentane) as if they had been given LSD.
Dr Nichols:
3-aminopentane has a potency in the drug discrimination behavioral assay of 52 nanomoles per kilogram of rat body weight, this is very similar to the 48 nanomoles per kg of rat body weight observed with LSD.
Last 3 pics: Home grown fresh potent mg seeds....if you can't grow them yourself, buy them on-line in bulk and store in freezer to keep potent until you use...also shown: the priest may have made LSI from claviceps paspali ergot (same alkaloid profile as the mesoamerican morning glory) as it grows it the famous Rarian plane adjacent to Eleusis, and served it for 2,000 years in ancient Greece to the psychedelic initiates (hundreds of people drank it at once every Sept). The Kykeon brew was known to contain ergot and fresh peppermint (contains isovaleraldehyde) all mixed together for some time. This easily made brew could have easily fed hundreds of people.

























Other topics: Alchemy chemistry fun:
Compilation of pan cyan or panaeolus cyanescens or copeandia cyanescens trip reports, crown jewel of mushrooms:
https://www.shroomery.org/forums/showflat.php/Number/28108398/page/1
How to extract 2.4g dmt from 170g bark using a 2 Liter erlenmeyer flask (heat and break resistant), post #15:
https://mycotopia.net/topic/111610-hpbcd-dmt-sublingually-active-under-tongue/
Tetrahydroharmine or THH and how to make her, Caapi visionary feminine teaching spirit:
https://www.shroomery.org/forums/showflat.php/Number/28423951/page/1
Zero nausea HPBCD or aloe vera enhanced penetration Ayahuasca capsules:
https://www.shroomery.org/forums/showflat.php/Number/28189371/page/1
Cactus tea before waterpark to beat the heat:
https://www.shroomery.org/forums/showflat.php/Number/28411312/page/4



How to make LSI or Lysergic Acid Isovaleraldemide (Greek Eleusis ancient LSD) at home from morning glory seeds (the priests used non poisonous claviceps paspali which grows on paspalum grass adjacent to Eleusis present day in the famous Rarian plane, same alkaloid profile as the sacred Mesoamerican morning glory):
https://www.shroomery.org/forums/showflat.php/Number/27850299/page/2
Make your own 1-acetaldehyde LSD at home from LSD, very similar to ALD-52 or the real orange sunshine:
https://www.shroomery.org/forums/showflat.php/Number/28441105
On my very first pan cyan mushroom trip, where I went to a house music club tripping with friends, I viewed laser light patterns on the floor of the club, where the women danced, I believe the mushrooms showed me how to form never before seen patterns, as went I went home, over the next several months, I built my own 6 channel audio generator that when these combined frequencies (3 on x channel and 3 on y channel) were sent to a laser x and y galvanometer, were able to produce brand new laser patterns such as collapsing circles and spinning lines 360 degrees which looked beyond belief in the fog as 3-d, I then went on to market these laser scanners to clubs on the strip, and they were a huge success...I owe this creative invention to the mushrooms which sparked new creative energies, way beyond thought, from a higher source where the mushrooms tap into. My love for house music stems back to those days of visiting many clubs as an entertainment laser lighting fixture creator and programmer and making friends with the many DJ's. Over the summer myself and friends were lifeguards at the local water park. But on the weekends we went to parties or house music clubs.
https://www.friskyradio.com/
https://jaytechmusic.podbean.com/
I've had numerous pm's to condense the tek onto 1 page with pics, as lots of people are wanting to try this, so here it is...print this out and carry it with you and study it, it's super easy and fast I promise.
COMPLETE TEK ON HOW TO MAKE LSH and LSI combo or just LSI (your preference) on this one page with supporting studies, very simple:
Self trip with 600 seeds:
If you only have a few HBWR seeds, you can mix them for a few minutes with pure peppermint oil drops, see post #1 for recommendation on how many drops of pure peppermint oil to use, or just read below.Quote:
LSI trip report made from MG seeds: on my 600 seed or 18g high dose morning glory wine extract prepared with 3 drops isovaleraldehyde and 1 teaspoon apple cider vinegar as catalyst for the aldehyde condensation onto LSA to form LSI, saw 2 hours of dancing colored geometrics, thousands upon thousands they were so intricate for 2 hours which formed completely naked women goddesses at the intersections of the geometrics, mind blowing music enhancement and euphoria, very strong trip for 6 hours with 8 hour duration. One of the most prominent visual features is the seeing of colored energy fields or auras surrounding all objects just as itbebasidia reported, I see this every time the whole trip. Very powerful shimmering and glowing of everything as well, as if there is an intense divine light inside, very beautiful. Mentally: Deep healing head space.
This trip report from Kash (long time LSA master) below is taken from post #3 of the beginning of this thread....keep in mind that LEMON JUICE also contains isovaleraldehyde, my best trip report using just a few HBWR was when I mixed them with fresh lemon juice from the fridge for a while, then consumed.
Do not use the cheap grocery store peppermint alcohol extract, instead buy real Peppermint oil max dosage: Adults: 0.2 to 0.4 mL (4 drops to 8 drops max for adults, DO NOT EXCEED: https://www.aafp.org/pubs/afp/issues/2007/0401/p1027.html
I still don't recommend using HBWR but it will still work to form LSI, see post #2 at very beginning of this thread: all about why this is so (MG vs HBWR seeds)...the main reason is that HBWR contains high amounts of ergometrine which causes cramping and vasoconstriction...whereas MG only has traces of it, I've used MG up to 25g and still no cramping or vasoconstriction. You can get way higher on MG seed LSI whereas with HBWR you will start to experience the somatic side effects of cramping way sooner due to the ergometrine if you are not careful on dosage, it's a balancing act with HBWR.
Psychonauts (psychedelic astronauts): you can still find isovaleraldehyde out there, you just have to look, try looking for the 25ml bottle.
Kash:
p.s if you read post #2, you will see that ALL the best MG trip reports involved adding fresh lemon juice, examples below, this is because LEMON JUICE ALSO contains isovaleraldehyde:Just took a 40 seed portion of LSA extract that was mixed for 15 minutes with peppermint oil (contains 2mg acetaldehyde per 5 drops, editors note: and contains even more isovaleraldehyde) yesterday and tripped his face off with a friend. Was very clean feeling and relaxed. Rainbows and vibrant fractal energy danced all over the skies and throughout his surroundings and music sounded great. The head-space was very acid like but different. Was a bit intense but he was able to keep it together lol. Whole trip was about 8 hrs long.
I have tried a 20 seed extract without peppermint oil and it seemed uncomfortable and sedating with no visuals, while every time he has added peppermint oil he has gotten visuals.
vespiary:
Hermes (Lycaeum):Quote:
Having read someone's description of a cold water extraction with added lemon juice, I decided to try it one day. I've tried this method several times and will describe my best trip to date following these notes…
I've found that the ideal dose for my body weight (5'9, 200 lb.) is 30 grams of seeds. I TRIPPED HARD AS HELL.
I saw geometric patterns in everything…
It ranked up there with the best trips of my life, and that includes Mescaline, LSD, and Mushrooms. The trip lasted for about 8 hours and seemed to peak at about 3 hours. Unlike an acid trip where I peak rapidly and then come down fast, this is more like I peak slowly, level off, and then come down slow. I am always in control of my faculties.
My comment: Lemon juice also contains ISOVALERALDEHYDE just like peppermint leaf.Quote:
With a 400 morning glory seed extract into cold distilled water with a squirt of lemon juice, I see amazing three and seemingly four-dimensional shapes morphing and bifurcating. Often I get religious and esoteric themed visuals, like fractal cherub wings and winged eyes like those in some of Alex Grey's work. Eyes are all over everything! I see pyramids and sphinxes and Gigeresque biomechanical forms. I see amazing geometric lattice structures. I watch mathematical space-filling algorithms doing their thing.
I also find that I can control the imagery by an act of will. Anything I intend to visualize comes forth and then goes beyond what I had imagined and then transforms into something else. This is great for artists! I could swear that on one occasion while listening to Mozart's Requiem, Heaven itself opened up inside my skull! It was incredible! OH, THE BEAUTY! I saw glorious celestial architecture and there were seraphs singing along with the chorus in the music. I just can't even begin to describe what this was like.
I had tears streaming down my face. I was in the highest ecstacy I have ever known. And all of this with a nothing more than a good mindset, good music, and only about 500 seeds. I'll never forget it. It was probably all in my imagination, programmed by my Catholic upbringing. I don't know and don't really care where it came from. It was the most beautiful thing I have ever experienced, and I still feel great joy when I think of it. I felt so whole!
Hermes (lycaeum March 2003) on morning glory visuals & strength, extracted into cold distilled water with a squirt of lemon juice:
My comment: lemon juice contains isovalearaldehyde as well.Quote:
Depending on seed quality, you should get some weak to decent effects from 8 grams, like mild reality bending, marked enhancement of aesthetic perception, insights, weak open eye visuals, moderate but subtle closed eye visuals (especially with music). I find that more satisfactory results are achieved with more seeds, like 14 grams or more. This much is needed to enter truly psychedelic territory. I wouldn't recommend this much if you haven't tried lower doses with the same seeds and method, however.
I usually just measure by Burpee bags. These are 1.8 grams each. I have used anything ranging from 3 to 12 of these. Strangely, the 12 bag trip was relatively weak. They must have been some old seeds. My strongest experience ever with MG seeds was with the Martha Stewart brand from K-Mart. I had strong effects, with good open eye visuals, on only 300 seeds! I used a cold water extraction with some added lemon juice, but the alcoholic extract by Stretchman also here at the Lycaeum I tried was the strongest.
I had strong 4D lattice-like open eye visuals and warping and melting of furniture with only 400 seeds. There are about 32-36 seeds to a gram. The Martha Stewarts are more expensive than the Burpees. They are like $1.30 a bag or something close to that for a 1.2 gram bag. The Burpees are something like $0.88 a bag and each bag has 1.8 grams. That's $2.05 a gram for Martha Stewart seeds and $0.92 a gram for Burpee seeds. It seems to be a trade off.
Nogal (the Nook):
My comment: lemon juice contains isovalearaldehyde as well.Quote:
Yes I know of someone who tried the CWE method with the Heavenly Blue variety, except with the substitution of a coffee grinder in place of a stone metate (I think that's what is called but I could be wrong), and a squirt of lemon in the water, with around 400-500 seeds. Closed and open eyed visuals were extremely breath taking. Some of the most prominent visions were of Aztec/Mayan glyphic patterns, a menacing and demonic technicolor nymph made of light who tried to seduce the viewer, and this bizare trail of energy spheres which each contained a different stylized animal form (again definately of Aztec/Mayan origin).
dmthead420:
Norman, mycotopia, said on 16 September 2019:Quote:
Seems this does do alot more, its alot more refined, clean, less body high all mind high.. i extracted 700 riveas into 100 ml of lemon juice , 50ml water .. that sat 9hrs in the fridge(water stayed the color of lemon juice but smelled like alkaloids) i filtered and added 100ml of sherry wine and that sat 6hours..
A buddy and i sampled 12ml of this and the effect is way different from just eating the seeds or just a simple water extract..
No body feelings AT ALL, not even the normal body buzz.. just a extreme lsd like head and abstract thoughts, better sense of understanding.... Real soon i am def going to try a large dose ..I Feel GreaT...I will no longer do it any other way.....my friend says the same.
Red22:Quote:
Years ago I stumbled across a simple method for dosing HBWR.
Grind the seeds and cover them with white wine, let sit in the fridge for a day or so, shaking occasionally, decant, filter and drink.
No nausea no aches no vasoconstriction.
I am now off alcohol completely so I’m thinking of an alternative method short of a full on extraction.
I’m convinced that something in the wine besides water and alcohol is what makes the trip so clean. I’ve tried twelve percent water alcohol mixes in the past and still had the nasty side effects and at the same time the trip is not as strong.
I’m thinking acetaldehyde and or tartaric acid may be involved or at least a good place to start.
Any thought on what chemically may be going on?
Quote:
"This paper states tha "LSH" was identified in blood and/or urine samples in two individuals who ingested the seeds. This is interesting because Peter Webster alleges that LSH will immediately decompose when in the body. This paper indicates that that's not true.
Klinke HB, Müller IB, Steffenrud S and Dahl-Sørensen R, Two cases of lysergamide intoxication by ingestion of seeds, which resulted in one fatality due to falling from a building and one surviving witness. Forensic Sci Int, 2010, 197(1-3), e1-5. https://pubmed.ncbi.nlm.nih.gov/20018470/

Easy steps, I've used this process over two dozen times in a 2 year period, it works with zero nausea:
---> You can even drink this LSH and LSI enhanced liquid extract at the same time you drop your LSD for an experience that is way beyond LSD, the LSH and LSI adds infinite dimensions to the normal LSD experience, like incredible (I mean phenomenal !) music enhancement, every sound in the music is "epic", infinite beauty enhancement, strong sensual feelings, strong neon like color saturation, flowing visuals, energy fields seen in constant motion around all objects, heavy tracers, etc. These qualities LSH and LSI adds are very similar to what cactus tea adds in my experience when added to LSD.
This is most likely due to LSH and LSI hitting 5 more adrenal recpetors associated with beauty, aesthetics and sensuality beyond normal LSD, see radioligand chart for LSH. I used this potion every time I tripped, you can even add it to cactus tea by taking drinking the LSH potion 1 hour later (always stagger 1 hour so the amides do not clash with the trace maoi's in cactus, only take in that order) it's incredible.
------------------------------------------------------------------------------
--> How to make the ZERO NAUSEA morning glory wine which is used for all aldehyde experiments, example: forming LSH and LSI from the LSA in the seeds: <--
How this works, from 2022 aldehydes paper, see 1960's study by Arcamone, go to post #1 to read all the studies (5 papers). LSH and LSI do make it to the bloodstream and urine, so it does indeed reach the brain so long as you keep it in it's original state (in acidic wine so it does not decompose back to LSA which will happen in plain water or nonacidic solutions) store in fridge or freeze in freezer to keep forever, drink as so.
Pic 1: Sherry wine is the wine highest in acetaldehyde.
Pic 2: With only a lamp on in garage, no overhead bright light: grind 15 grams or 525 heavenly blue morning glory seeds in a coffee grinder (35 seeds per gram) using 10 second grind with occasional shaking of coffee grinder, pause 5 seconds, then grind again 10 seconds, repeat x 4 times, you will end up with a dust like consisteny, all the alkaloids are extracted from within the tough rubbery embryo of the seed. 525 seeds x .01mg LSA per seed = 5mg LSA
Pic 3: Place ground seed dust into a 1/2 pint tall jar (*almart case of 12, canning section), add 4 shots of cold just opened fresh sherry wine from the fridge, and around 30mg of DL tartaric acid which aids the extraction (from hi media store, *mazon, auction or similar). Do not use plain L-tartaric acid used in wine making, get the good stuff, which contains the D isomer. D-lyergic acid amide isomer salts are the potent form of salts.
And also ADD a tiny splash or 1 to 2 teaspoons of acetic acid or VINEGAR (I use apple cider vinegar) to catalyze the condensations of the aldehyde to LSA: acetaldehyde in this case onto the amide of LSA, and isovaleraldehyde to form LSI. The vinegar acts as an important catalyst as it contains acetic acid, and speeds the formation of new adduct product, see study below:
From Mehra, R.K & Panya, K.C. 1938, The condensation of aldehydes with amides - part III, the condensation of cinnamaldehyde with acetamide. Proc Natl Acad Sci India Phys Sci 7(6). 376-380
NOTE: from paper: Acetamide (very similar to LSA) when cinnamaldehyde adduct added, forms: N,N'cinnamylidenediacetamide just as LSA + isovaleraldehyde forms LSI or Lysergic Acid Isovaleraldemide. RATS RESPOND TO it as if they had been given LSD, and this human rat loves the effects, the closest thing in nature I've ever experienced that is very similar to LSD. LOVE, LOVE, LOVE !!!



Cinnamaldehyde should not be used as it contains nearly twice the number of carbons and hydrogens and thus weak activity due to this (weights way too much)...only use isovaleraldehyde (weight is perfect) to form the potent LSI. However, I am showing the study so that you see an even "longer chain aldehyde than isovalearaldehyde" will even adduct onto acetamide or LSA as proven in study.
YOU WILL LOVE LSI, it is so very similar to LSD !!!



Re-seal your sherry wine by spraying wine preservation canister which has inert gas (contains argon, carbon dioxide, etc.) around ten dollars from *mazon into your wine before sealing with cork and placing back in fridge, this way the precious acetaldehyde in the wine will not oxidize to vinegar as normally happens over a 5 day period when corked without preserving.
Put lid on and shake contents for 1 to 2 minutes.
Place jar into fridge for 10 minutes after the 2 minute shaking, during this time the nauseating seed debris can be seen falling to the bottom.
Pic 4: Filter entire contents of jar thru a "coffee wire filter" from *almart or *amazon or your local grocery store sitting atop a glass, the liquid above the seed debris will filter real fast in seconds, then filter the debris at the bottom from the jar, once the debris is in basket, use a spoon to press down on it to get all the liquid out, this only takes around 10 seconds.
Pic 5: Place this filtered liquid in the fridge for 3 hours or even overnight, within 3 hours, all of the nauseating to the intestines seed debris will have fallen to the bottom. This process begins within an hour after sitting in fridge, check each hour and watch as the seed debris falls by around an inch each hour.
Pic 6: After 3 hours or longer, decant the liquid from above the bottom 1/4" of seed debris, this is what you want to consume, it is psychedelic and results in zero nausea, as there is no seed debris in it. DO NOT DRINK the SEED DEBRIS at very bottom or you will become nauseated and sick, don't worry, there are no alkaloids in it.
Note: never try to extract your seeds using just plain acidified water, I have tried this before, your seed debris will not separate out in the fridge, and you end up with a complete emulsion in the coffee wire filter which will not filter at all, wine is needed to effect the proper extraction, and allows the liquid to separate from the seed emulsion below, this will not happen with a plane water extration. This is how the ancient Aztec and Mayan extracted the seeds using balche, an alcohol they made themselves, see 2nd pic.
Rest of the pics:
sources of isovaleraldehyde: pure chemical (on line 25ml bottle) or you can use fresh lemon juice or pure peppermint oil, I prefer to use the pure chemical.
Use ice bath on your stirrer to keep wine as a combo of LSH + LSI (as LSH adduct acetaldehyde boils off at room temp, so always keep cold, to form LSI only, omit the ice bath, as LSI does not boil off till 198 degree F).
All the alkaloids are concentrated in the fridge decanted liquid above the seed debris. This contains your LSA which has adducted to the acetaldehyde in the wine, forming new amounts of LSH, since the wine is at ph=4, the new LSH adduct product will remain stable indefinately, store in fridge if you plan to use within a few days, or you can freeze and de-thaw in fridge overnight to use next day. It dethaws fast since it is wine.
You just formed LSH using the method above, to form LSI, simply take your 4 shot morning glory wine, and at this point add your 3 drops of isovaleraldehyde along with 1 to 2 teaspoons apple cider vinegar (if you don't have the pure chemical then add teaspoon or more of lemon juice, or several drops of pure peppermint oil) to form new molecule similar to LSD in every way. Be sure to spin for around 1 hour at high speed. I always use the pure chemical as I have it. Don't forget to add the apple cider vinegar as it contains the acetic acid catalyst which speeds up the adduct chemical reaction.









-----------------------------------------------------------------------------------------------------
References: Lysergic acid Isovaleraldemide, LSI
The aldehyde in peppermint oil and leaf and lemon juice is Isovaleraldehyde otherwise known as 3-methylbutanal. If you are a business or researcher you can order it directly, there are also two places that sell direct to individuals, just google it. They delivered it straight to my door, one place starts with a "*******" All you need to add is two drops of this to your LSA sherry wine liquid extract as it spins on the stir mantel for 1 hour at high speed, also must add 1 teaspoon of either vinegar or apple cider vinegar (my preference), as the acetic acid in the vinegar is the catalyst for the condensation of the isovaleraldehyde onto the LSA forming Lysergic Acid Isovaleraldemide, see study above.
There is .01mg LSA in 100 seeds (.01 x 100 = 1mg LSA), just a 100 seed extract has effects very similar to 100ug of LSD, LSI is 1/10th the potency of LSD. Immense visual power with closed eye geometrics, wild colors, music sounds bad ass, divine healing power. I have prepared an extract with 200 seeds and the strength increases similar to 200ug of acid.
I have been tripping my ass off every 14 days or so taking this LSI liquid extract around 1 hour after a very small bridgesii tea prepared with only 450 grams of bridgesii cactus chuncks from around the core (around 225mg mescaline), as I love the combo of cactus + LSD, this is no different. Yes, I have tried LSI many times without the cactus, super potent, highly recommend. I will be using LSI for the rest of my life.
This aldehyde is completely safe and has been administered in high does to rats with no ill effects. This aldehyde as you can see from paper makes up a substantial part of peppermint oil and leaf.
More on LSI:
chemical formula for entry #16....3-aminopentane = CH(C2 H5)2
chemical formula for isovaleraldehyde is (CH3)2 CH2 CH2 CHO
Notice isovaleraldehyde even LOOKS EARILY similar to the tale end of psilocin or DMT with nearly the same exact chemical formula as the tail end of psilocin or DMT, see pics in middle

diethylamine from LSD has 4 carbon groups and 11 hydrogen groups with a molecular weight of 73 g/mol.
Both the 3-aminopentane and the isovaleraldehyde of this new discovery have 5 carbon groups and 11 hydrogen groups (once the aldehyde attaches to the H already at the amide of LSA) and BOTH have exact same molecular weights of 87 g/mol similar to diethylamine molecular weight of LSD at 73g/mol.
The 3-aminopentane from Dr. Nichols and isovaleraldehyde discovery join at the R1 substitute of LSA with R=H still. This new aldehyde has same molecular weight as 3-aminopentane on the R1 substitute of LSA with R=H, see Dr. Nichol's paper on page 84, chart shown. This is one of only 2 entries in which the rats responded to it (3-aminopentane) as if they had been given LSD.
Dr Nichols:
Quote:
The important thing to note from the table below, in the far right column, is the fact that LSD has a potency in rats in the drug discrimination behavioral assay of 48 nanomoles per kilogram of rat body weight. Only two other compounds have comparable activity: entries 6 and 16.
Curiously, entry 6 is a monoalkylamide that has the same molecular weight as LSD itself, that is, it has a total of four carbon atoms attached to the amide. Entry 16 has a five-carbon group attached to the amide.
We have no evidence as to whether either of these compounds would be active in man, but these rat data suggest that they might be.
3-aminopentane has a potency in the drug discrimination behavioral assay of 52 nanomoles per kilogram of rat body weight, this is very similar to the 48 nanomoles per kg of rat body weight observed with LSD.
Last 3 pics: Home grown fresh potent mg seeds....if you can't grow them yourself, buy them on-line in bulk and store in freezer to keep potent until you use...also shown: the priest may have made LSI from claviceps paspali ergot (same alkaloid profile as the mesoamerican morning glory) as it grows it the famous Rarian plane adjacent to Eleusis, and served it for 2,000 years in ancient Greece to the psychedelic initiates (hundreds of people drank it at once every Sept). The Kykeon brew was known to contain ergot and fresh peppermint (contains isovaleraldehyde) all mixed together for some time. This easily made brew could have easily fed hundreds of people.

























Other topics: Alchemy chemistry fun:
Compilation of pan cyan or panaeolus cyanescens or copeandia cyanescens trip reports, crown jewel of mushrooms:
https://www.shroomery.org/forums/showflat.php/Number/28108398/page/1
How to extract 2.4g dmt from 170g bark using a 2 Liter erlenmeyer flask (heat and break resistant), post #15:
https://mycotopia.net/topic/111610-hpbcd-dmt-sublingually-active-under-tongue/
Tetrahydroharmine or THH and how to make her, Caapi visionary feminine teaching spirit:
https://www.shroomery.org/forums/showflat.php/Number/28423951/page/1
Zero nausea HPBCD or aloe vera enhanced penetration Ayahuasca capsules:
https://www.shroomery.org/forums/showflat.php/Number/28189371/page/1
Cactus tea before waterpark to beat the heat:
https://www.shroomery.org/forums/showflat.php/Number/28411312/page/4


How to make LSI or Lysergic Acid Isovaleraldemide (Greek Eleusis ancient LSD) at home from morning glory seeds (the priests used non poisonous claviceps paspali which grows on paspalum grass adjacent to Eleusis present day in the famous Rarian plane, same alkaloid profile as the sacred Mesoamerican morning glory):
https://www.shroomery.org/forums/showflat.php/Number/27850299/page/2
Make your own 1-acetaldehyde LSD at home from LSD, very similar to ALD-52 or the real orange sunshine:
https://www.shroomery.org/forums/showflat.php/Number/28441105
On my very first pan cyan mushroom trip, where I went to a house music club tripping with friends, I viewed laser light patterns on the floor of the club, where the women danced, I believe the mushrooms showed me how to form never before seen patterns, as went I went home, over the next several months, I built my own 6 channel audio generator that when these combined frequencies (3 on x channel and 3 on y channel) were sent to a laser x and y galvanometer, were able to produce brand new laser patterns such as collapsing circles and spinning lines 360 degrees which looked beyond belief in the fog as 3-d, I then went on to market these laser scanners to clubs on the strip, and they were a huge success...I owe this creative invention to the mushrooms which sparked new creative energies, way beyond thought, from a higher source where the mushrooms tap into. My love for house music stems back to those days of visiting many clubs as an entertainment laser lighting fixture creator and programmer and making friends with the many DJ's. Over the summer myself and friends were lifeguards at the local water park. But on the weekends we went to parties or house music clubs.
https://www.friskyradio.com/
https://jaytechmusic.podbean.com/
Last edited: