-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,483

THE_CRYSTAL_METHOD
1-(4-methanoneylindole-3-yl)-2-dimethylaminoethane

THE_CHEMICAL_BROTHERS
1-(5-methanoneylindole-3-yl)-2-dimethylaminoethane

OWSLEY
1-(4-methanoneyl-2,5-dimethoxyphenyl)-2-aminopropane

TINY_TIM
1-(4-methanoneyl-2,5-dimethoxyphenyl)-2-aminoethane

ATLienz
1-(2,4,6-trimethanoneylphenyl)-2-aminopropane
Last edited:
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Without access to someone who is selling said mutant, it's a handy footnote, but don't expect to find it in your local Home Depot.Supposedly, there is at least one highly valued claviceps paspali mutant that produces direct LA. Was also told it yields 3g of LA per liter of culture every 10 days.
One journal article details the genetic manipulations needed to knock out a gene called IpsB, which is responsible for turning lysergic acid into ergometrine. It's not kitchen chemistry. They claim 3.7g of a mix of lysergic acid and isolysergic acid per liter of culture per 16 days.
The problem is classically more complex than other extractions.It cant be that difficult? Most natural products just require specific extraction and purification protocols.
Cannabis has a large amount of the psychoactive component present as a trivially separable form, and as the main constituent (Namely, THC-COOH and THC). Meaning even a simple solvent rinse and heat treatment is enough to isolate THC in workable purity.
Ergot on the other hand is far more aqueous and dilute than Cannabis. And proportionally it doesn't produce as much active component. What it does produce is usually a complex mixture of more polar compounds that are also more sensitive to things like oxygen and light.
One Hungarian patent claims about 100L culture = 10kg mycelium = ~100g crude ergot alkaloid. Mind you they also use more than 50L ethyl acetate as well as ammonia, phosphoric acid, a fluidized bed dryer, and chromatography over silica.
Doable? Yes. Easy as THC extraction? Not by a long shot. Also there is the small matter of growing the C.paspali in the first place,
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
I'll print all your structures on an A4 sheet each, put them in boxes and drop them on you from height.That I Can't Feel My Own Drugs?
You'll feel them then.
[e]
No, my father went by that. You can call me sekio, thanks. I believe the polite response to such an introduction, seeing our disparate social standings, would be to genuflect. And grovel a little too, I like that.sekio, (Can I Just Call You Dave?)
Last edited:
Important note: isovaleraldehyde used to be widely available even on *mazon and *igh media, (extracted or synthesized in India) but now only a couple places have it for sale directly to individuals, search for 25ml bottle, other places only sell to businesses or university. I am sorry it's not widely available, it is found in mint leaf and steam distilled mint oil. A 25ml bottle (around the price of 2 to 3 movie tickets) has 500 drops, so 2 drops per dose you add and spin with your morning glory wine for 1 hour at high speed on your stir mantel with 1 to 2 teaspoons of apple cider vinegar (contains acetic acid which is the chemical catalyst for the aldehyde adduction) will last around 250 doses or a lifetime.
See post #1 downloadable attachment in references section at the end of paper, for the study that even back in 1937 showed cinnamaldehyde (an even longer chain aldehyde than isovaleraldehyde) adducts onto LSA like looking molecules, and remains stable.
Forensic tests have shown that LSH (LSA + acetaldehyde adduct = LSH) does indeed reach the brain and was found in bloodstream and urine of 2 teenagers, so it does remain stable and reach the brain: https://pubmed.ncbi.nlm.nih.gov/20018470/
...and yes LSI does indeed also remain stable and reach the brain. The study even showed that a catalyst was not even needed to work with cinnamaldehyde, but I use a chemical catalysts (acetic acid in vinegar) to speed up reaction and guarantee it's success anyways, just like the studies I posted on post #1.
Convert ml to drops: https://www.unitconv...ter-to-drop.htm
LSA + isovaleraldehyde = Lysergic acid Isovaleraldemide.
I will post more trip reports in future.
----------------------------------------------------------------------
Having an ultra bad ass time tonight, 4 hours in...combined the zero nausea morning glory wine made with 25 grams heavenly blue seeds and several drops isovaleraldehyde with 3 hits of LSD, felt like 6 hits of acid and all the alkaloids in the morning glory seeds made it feel ultra-natural with intense shimmering of all visuals, deep insights, divine healing. The 6 other alkaloids in mg add so much more to the experience..very unique.
I never take LSD unless I combine it with at least 20 grams plus of morning glory wine, feels so much more like a high dose natural version of LSD, hits all the extra adrenal receptors that LSD normally does not hit, (same ones mescaline hits) this makes it extra colorful, no man made feeling at all, music is ultra enhanced way beyond just LSD. The shimmering of visuals is to die for. I like to combine the MG wine with LSD, cause I have lots of LSD and need to use it up. Been enjoying the tunes of jaytech all night.
If you need help making the zero nausea mg wine just go to page 11, and go half way down and follow the easy steps with pics: https://www.shroomery.org/forums/showflat.php/Number/27850299/page/2/fpart/11/vc/1

 jaytechmusic.podbean.com
jaytechmusic.podbean.com
LSI, page 1 with over dozen pics: https://www.shroomery.org/forums/showflat.php/Number/27850299/fpart/1/vc/1
See post #1 downloadable attachment in references section at the end of paper, for the study that even back in 1937 showed cinnamaldehyde (an even longer chain aldehyde than isovaleraldehyde) adducts onto LSA like looking molecules, and remains stable.
Forensic tests have shown that LSH (LSA + acetaldehyde adduct = LSH) does indeed reach the brain and was found in bloodstream and urine of 2 teenagers, so it does remain stable and reach the brain: https://pubmed.ncbi.nlm.nih.gov/20018470/
...and yes LSI does indeed also remain stable and reach the brain. The study even showed that a catalyst was not even needed to work with cinnamaldehyde, but I use a chemical catalysts (acetic acid in vinegar) to speed up reaction and guarantee it's success anyways, just like the studies I posted on post #1.
Convert ml to drops: https://www.unitconv...ter-to-drop.htm
LSA + isovaleraldehyde = Lysergic acid Isovaleraldemide.
I will post more trip reports in future.
----------------------------------------------------------------------
Having an ultra bad ass time tonight, 4 hours in...combined the zero nausea morning glory wine made with 25 grams heavenly blue seeds and several drops isovaleraldehyde with 3 hits of LSD, felt like 6 hits of acid and all the alkaloids in the morning glory seeds made it feel ultra-natural with intense shimmering of all visuals, deep insights, divine healing. The 6 other alkaloids in mg add so much more to the experience..very unique.
I never take LSD unless I combine it with at least 20 grams plus of morning glory wine, feels so much more like a high dose natural version of LSD, hits all the extra adrenal receptors that LSD normally does not hit, (same ones mescaline hits) this makes it extra colorful, no man made feeling at all, music is ultra enhanced way beyond just LSD. The shimmering of visuals is to die for. I like to combine the MG wine with LSD, cause I have lots of LSD and need to use it up. Been enjoying the tunes of jaytech all night.
If you need help making the zero nausea mg wine just go to page 11, and go half way down and follow the easy steps with pics: https://www.shroomery.org/forums/showflat.php/Number/27850299/page/2/fpart/11/vc/1

Jaytech Music Podcast | jaytechmusic
A series of mix shows from Australian DJ and producer Jaytech.
LSI, page 1 with over dozen pics: https://www.shroomery.org/forums/showflat.php/Number/27850299/fpart/1/vc/1
Last edited:
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
OK, real chemist dropping some truth bombs here. Tired of some of these age old rumours with zero proof.
If you think that imine formation is going to occur in aqueous environments, maybe Journal of Organic Chemistry would like to hear your methodology
It's an equilibrium reaction, amine + aldehyde <-> imine + water. If there is an abundance of reactants on one side, it will want to equalize both sides. So if a lot of water is around, any imine that forms will most likely bump into water and revert to amine+aldehyde again.
But wait a second, I missed something. LSA is an amide and not an amine. Amides are way less basic than amines and have totally different reactivity. That is why alpha-hydroxyethyl LSA doesn't just fall apart like an imide would- it's not an imide.
Part of the problem here is that while, yes, technically speaking LSH is an adduct of LSA and acetaldehyde, studies only ever show the adduct being decomposed to LSA and acetaldehyde, and never created.
I can say that THC-COOH is an adduct of CO2 and THC, and indeed, if you heat/acidify it, it decomposes to CO2 and THC. But no matter how much CO2 you add to THC you will never reform THC-COOH (in fact supercritical CO2 is used to extract THC...)
I cannot find any references to alpha-hydroxyalkyl amides forming spontaneously in solution (or even at all). Putting things on amides is a lot tougher than putting things on amines. (Wonder why we don't go from LSA to LSD directly? That's why) There is an OrgSyn paper making a 2,2,2-trifluoro-1-hydroxyethylamide from an amide and 2,2,2-trifluoro-1-methoxyethanol. but the analogous reagent to make a plain 1-hydroxyethylamide (1-methoxyethanol)... is not a bench stable compound, as a hemiacetal of acetaldehyde. (Also the reaction does not proceed with the aldehyde, it needs the hemiacetal.)
You would figure that if the reaction between LSA and acetaldehyde was as quick and easy as it is claimed, that I would be able to find plenty of other references and papers of similar reactions occuring, between an aldehyde and an amide in aqueous solution. But I can't find anything aside from this repeated playground rumour of LSA forming adducts.
There has never been a proven literature synthesis of LSH from LSA. I am willing to be proven wrong with any journal article that clearly demonstrates a plausible reaction pathway and can demonstrate formation of LSH with at least chromatographic evidence (either isolation of the pure material, or just a mass spec from a mixture on LC or GC). This means no trip reports, no hypothetical wishy washy shit. I want to see hard proof.
I might even send a trinket or postcard to anyone who does so as a prize for scientific achievement in drug sciences. (I will give a consolation prize to anyone showing a proven synthesis of an alpha-hydroxyalkyl amide (any synthesis at all) or anyone who can, in a double blind study with preferably more than 10 peopls, prove that LSA and LSA+aldehyde is actually any different than LSA.)
I also have serious doubts that anyone with a head full of acid would be able to effectively judge the effects of, well, any added drugs. Especially if they verge on placebo. LSD makes people more suggestible so it's hard to take any claims based exclusively on how you feel when you're, y'know, high on acid.
The one semi reliable reference from those papers that references a reaction between an amide and an aldehyde is an Indian Academy of Sciences from 1938 that apparently nobody (including you) actually read fully. They make some freak compound of two amides and one aldehyde instead of an imide or hydroxyethylamide though. And their synthesis was not at room temperature, at millimolar dilution, in aqueous solution. They heat a neat mixture of pure amide, pure aldehyde, and fucking pyridine at temperatures between 100C and 160C for several hours to a day and get yields of between 6.2-50%. Also none of their amides used were even close to lysergamide in terms of complexity. LSA/ergine melts and decomposes at 135C. Also you will note that the catalyst used (pyridine) is basic not acidic, and not the kind of thing you want to eat. (Smells like rotting fish taint)
The other papers you linked are either mislabeled or total shit and do not form a body of evidence to support your claims at all.
"Has the Mystery of the Eleusinian Mysteries been solved .pdf" No. Next.
"DunningRobertL1956 (2).pdf" is someone's dissertation and presumably "evidence" of 3-methylbutanal in peppermint oil. If you spend 5 minutes to actually read the paper, it's pretty poor evidence. They are still using paper chromatography, and most importantly, seemingly cannot effectively seperate isovaleraldehyde from the menthol (oops). Also, they don't do any quantitative measurements (they don't determine how much isovaleraldehyde is present).
Another problem is that peppermint, like many plants, can exist in many differing chemotypes that have different compositions of essential oil. So, some peppermint oil may contain some, but other oils may not (and in my experience I have never seen isovaleraldehyde in peppermint oil, and I ran a GC doing terpene chemistry for ~5 years).
"3-methylbutanal or isovaleraldehyde safety sheet, low in general toxicity after oral exposure.pdf"' - Yeah, it's probably not super toxic, but it's a skin and eye irritant, and also smells strongly of toejam. It is not pleasant. (I would regularly use large volumes of isovaleraldehyde as a precursor to cryptone and 2-menthen-1-ol. Always used it in a fume hood. Would not consider eating it.) It is, however, rated as food grade for flavor and fragrance use from some suppliers.
"Chapter5_Excerpt_Peppermint.pdf" - Cool stuff about peppermint oil and its potential for allergies, but isovaleraldehyde does not appear in the list of typical components of ISO standard peppermint oil. It only indicates that one study found that there was isovaleraldehyde present in some samples, and didn't specify an amount. (Not quantitative.) Not very strong evidence.
"The LSA component of claviceps paspali forms LSH when dunked into fermented liquor (wine).pdf ", actual title "Production of a new lysergic acid derivative in submerged culture by a strain of Claviceps paspali", apparently never read the paper either (noticing a pattern here), because the "fermented liquor" is not wine, it's the fermentation liquid being used to grow the C. paspali! The paper talks about how they isolated a strain of ergot that naturally produces lysergic acid hydroxyethylamide exclusively, and says nothing about synthetic methods for making LSA into LSH.
"2016 Polish morning glory study shows LSH and penniclavine two highest alkaloids in MG (3).pdf" I don't have an issue with, I think it's established LSH is present in (at least fresh) morning glories/HBWR. (Penniclavine doesn't look active to me.) No claims for making LSH from LSA.
"5 Ergot alkaloids in morning glory just as stimulating as LSD in animal experiments, Yui and Yuji Takeo, 1958.pdf " again, no issue. We know that LSA is about 1/10 the potency of LSD in man, and LSH is presumably between LSD and LSA. Except I have to point out the obvious, animals are really, really bad at writing trip reports or describing body load. Does not describe LSH synthesis.
"Adduct formation of d-Lysergic Acid Amide or (LSA) and aldehydes.pdf" has the actual title of "The Theoretical Synthesis and in silico Modelling of Lysergic Acid Biscinnamylidene Amide from the Adduct Formation of d-Lysergic Acid Amide and Cinnamaldehyde" (translation: We never did any actual chemistry, We made some wild guesses, drew a structure, and plugged it into a web tool to predict activity)
It is the worst paper of the lot. There is nothing actually proven or discovered. Totally masturbation worthy of this very forum. It has such laugh-inducing content as:
** (They also give no reference to the mechanism that would form a hydroxyethylamide from LSA,)
The whole paper is basically them making hypothetical claims with zero actual evidence backing up the synthesis being possible.
"Tryptophan analogues form adducts by cooperative reaction with aldehydes and alcohols or with aldehydes alone, 1992 Austin.pdf" indeed shows compounds like indole acetic acid (which, wait a second, where's the amide to link up with there?) forming adducts with aldehydes.(imines). on the aromatic secondary nitrogen of the indole. Whoops. (No reaction at any amide groups shown.)
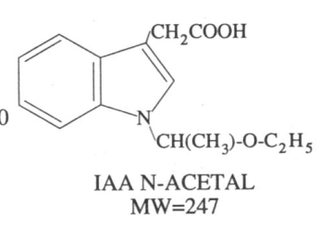
There's two ways you can respond to this. Consider all the evidence here and make a change, or continue believing rumours and whispers.
I'm sure taking LSD and morning glory together is lots of fun, but as a chemist with years of experience and a thing for dispelling drug legends, continuing to make claims that have never been verified in a reputable way (no isolation of LSH from LSA+whatever mixtures, not even a TLC plate which would take 15 mins and show a new compound forming quite clearly, costs minimal, can be done by average Jose). It's always bioassays and trip reports. Well, people are fallible. (even moreso with a head full of acid.) Consider Shulgin's famous anecdote:
This is like claiming the blotter art signifies different 'types' of LSD that produce different 'flavours' of trips. Or Cannabis sativa and Cannabis indica having differing effects (nowadays both are usually high THC low CBD cultivars). Or terpenes having major effects at all beyond smells. IME, all of these are old rumours that need to go away.
Otherwise I could mix my piss in your MG wine and claim that it makes a superactive hallucinogen from, I dunno, glucuronic acid or something, based upon solely my claim that "Well, I drank a 50/50 piss and MG wine mixture and had an intense and highly sexually charged trip that lasted 4 days." I could come up with a bunch of misread "evidence" papers too! But I don't because I'm trying to practice actual science and critical thinking.
If you think that imine formation is going to occur in aqueous environments, maybe Journal of Organic Chemistry would like to hear your methodology
It's an equilibrium reaction, amine + aldehyde <-> imine + water. If there is an abundance of reactants on one side, it will want to equalize both sides. So if a lot of water is around, any imine that forms will most likely bump into water and revert to amine+aldehyde again.
But wait a second, I missed something. LSA is an amide and not an amine. Amides are way less basic than amines and have totally different reactivity. That is why alpha-hydroxyethyl LSA doesn't just fall apart like an imide would- it's not an imide.
Part of the problem here is that while, yes, technically speaking LSH is an adduct of LSA and acetaldehyde, studies only ever show the adduct being decomposed to LSA and acetaldehyde, and never created.
I can say that THC-COOH is an adduct of CO2 and THC, and indeed, if you heat/acidify it, it decomposes to CO2 and THC. But no matter how much CO2 you add to THC you will never reform THC-COOH (in fact supercritical CO2 is used to extract THC...)
I cannot find any references to alpha-hydroxyalkyl amides forming spontaneously in solution (or even at all). Putting things on amides is a lot tougher than putting things on amines. (Wonder why we don't go from LSA to LSD directly? That's why) There is an OrgSyn paper making a 2,2,2-trifluoro-1-hydroxyethylamide from an amide and 2,2,2-trifluoro-1-methoxyethanol. but the analogous reagent to make a plain 1-hydroxyethylamide (1-methoxyethanol)... is not a bench stable compound, as a hemiacetal of acetaldehyde. (Also the reaction does not proceed with the aldehyde, it needs the hemiacetal.)
You would figure that if the reaction between LSA and acetaldehyde was as quick and easy as it is claimed, that I would be able to find plenty of other references and papers of similar reactions occuring, between an aldehyde and an amide in aqueous solution. But I can't find anything aside from this repeated playground rumour of LSA forming adducts.
There has never been a proven literature synthesis of LSH from LSA. I am willing to be proven wrong with any journal article that clearly demonstrates a plausible reaction pathway and can demonstrate formation of LSH with at least chromatographic evidence (either isolation of the pure material, or just a mass spec from a mixture on LC or GC). This means no trip reports, no hypothetical wishy washy shit. I want to see hard proof.
I might even send a trinket or postcard to anyone who does so as a prize for scientific achievement in drug sciences. (I will give a consolation prize to anyone showing a proven synthesis of an alpha-hydroxyalkyl amide (any synthesis at all) or anyone who can, in a double blind study with preferably more than 10 peopls, prove that LSA and LSA+aldehyde is actually any different than LSA.)
I also have serious doubts that anyone with a head full of acid would be able to effectively judge the effects of, well, any added drugs. Especially if they verge on placebo. LSD makes people more suggestible so it's hard to take any claims based exclusively on how you feel when you're, y'know, high on acid.
The one semi reliable reference from those papers that references a reaction between an amide and an aldehyde is an Indian Academy of Sciences from 1938 that apparently nobody (including you) actually read fully. They make some freak compound of two amides and one aldehyde instead of an imide or hydroxyethylamide though. And their synthesis was not at room temperature, at millimolar dilution, in aqueous solution. They heat a neat mixture of pure amide, pure aldehyde, and fucking pyridine at temperatures between 100C and 160C for several hours to a day and get yields of between 6.2-50%. Also none of their amides used were even close to lysergamide in terms of complexity. LSA/ergine melts and decomposes at 135C. Also you will note that the catalyst used (pyridine) is basic not acidic, and not the kind of thing you want to eat. (Smells like rotting fish taint)
The other papers you linked are either mislabeled or total shit and do not form a body of evidence to support your claims at all.
"Has the Mystery of the Eleusinian Mysteries been solved .pdf" No. Next.
"DunningRobertL1956 (2).pdf" is someone's dissertation and presumably "evidence" of 3-methylbutanal in peppermint oil. If you spend 5 minutes to actually read the paper, it's pretty poor evidence. They are still using paper chromatography, and most importantly, seemingly cannot effectively seperate isovaleraldehyde from the menthol (oops). Also, they don't do any quantitative measurements (they don't determine how much isovaleraldehyde is present).
Another problem is that peppermint, like many plants, can exist in many differing chemotypes that have different compositions of essential oil. So, some peppermint oil may contain some, but other oils may not (and in my experience I have never seen isovaleraldehyde in peppermint oil, and I ran a GC doing terpene chemistry for ~5 years).
"3-methylbutanal or isovaleraldehyde safety sheet, low in general toxicity after oral exposure.pdf"' - Yeah, it's probably not super toxic, but it's a skin and eye irritant, and also smells strongly of toejam. It is not pleasant. (I would regularly use large volumes of isovaleraldehyde as a precursor to cryptone and 2-menthen-1-ol. Always used it in a fume hood. Would not consider eating it.) It is, however, rated as food grade for flavor and fragrance use from some suppliers.
"Chapter5_Excerpt_Peppermint.pdf" - Cool stuff about peppermint oil and its potential for allergies, but isovaleraldehyde does not appear in the list of typical components of ISO standard peppermint oil. It only indicates that one study found that there was isovaleraldehyde present in some samples, and didn't specify an amount. (Not quantitative.) Not very strong evidence.
"The LSA component of claviceps paspali forms LSH when dunked into fermented liquor (wine).pdf ", actual title "Production of a new lysergic acid derivative in submerged culture by a strain of Claviceps paspali", apparently never read the paper either (noticing a pattern here), because the "fermented liquor" is not wine, it's the fermentation liquid being used to grow the C. paspali! The paper talks about how they isolated a strain of ergot that naturally produces lysergic acid hydroxyethylamide exclusively, and says nothing about synthetic methods for making LSA into LSH.
"2016 Polish morning glory study shows LSH and penniclavine two highest alkaloids in MG (3).pdf" I don't have an issue with, I think it's established LSH is present in (at least fresh) morning glories/HBWR. (Penniclavine doesn't look active to me.) No claims for making LSH from LSA.
"5 Ergot alkaloids in morning glory just as stimulating as LSD in animal experiments, Yui and Yuji Takeo, 1958.pdf " again, no issue. We know that LSA is about 1/10 the potency of LSD in man, and LSH is presumably between LSD and LSA. Except I have to point out the obvious, animals are really, really bad at writing trip reports or describing body load. Does not describe LSH synthesis.
"Adduct formation of d-Lysergic Acid Amide or (LSA) and aldehydes.pdf" has the actual title of "The Theoretical Synthesis and in silico Modelling of Lysergic Acid Biscinnamylidene Amide from the Adduct Formation of d-Lysergic Acid Amide and Cinnamaldehyde" (translation: We never did any actual chemistry, We made some wild guesses, drew a structure, and plugged it into a web tool to predict activity)
It is the worst paper of the lot. There is nothing actually proven or discovered. Totally masturbation worthy of this very forum. It has such laugh-inducing content as:
* (They give no reference for this. Peppermint leaves have almost no acetaldehyde, in fact peppermint oil is mostly menthol and menthone. Acetaldehyde is toxic, volatile, reactive, and has a very objectionable smell, from having worked with it.)This adduct formation is supported by anecdotal evidence of co-administering LSA with fresh peppermint leaves on forums [...] Fresh peppermint leaves are high in acetaldehyde*; thus, it is probable that individuals are forming LSH through the adduct formation of LSA and acetaldehyde**
** (They also give no reference to the mechanism that would form a hydroxyethylamide from LSA,)
The whole paper is basically them making hypothetical claims with zero actual evidence backing up the synthesis being possible.
"Tryptophan analogues form adducts by cooperative reaction with aldehydes and alcohols or with aldehydes alone, 1992 Austin.pdf" indeed shows compounds like indole acetic acid (which, wait a second, where's the amide to link up with there?) forming adducts with aldehydes.(imines). on the aromatic secondary nitrogen of the indole. Whoops. (No reaction at any amide groups shown.)

'Drops' are not a standard unit of any sort, and depends on the size of the dropper aperture, atmospheric temperature/pressure, the surface tension of the liquid, the specific dropper bulb, skill of the person using it, etc. Getting a 1mL syringe to do measurements is not going to break the bank.Convert ml to drops: https://www.unitconv...ter-to-drop.htm
There's two ways you can respond to this. Consider all the evidence here and make a change, or continue believing rumours and whispers.
I'm sure taking LSD and morning glory together is lots of fun, but as a chemist with years of experience and a thing for dispelling drug legends, continuing to make claims that have never been verified in a reputable way (no isolation of LSH from LSA+whatever mixtures, not even a TLC plate which would take 15 mins and show a new compound forming quite clearly, costs minimal, can be done by average Jose). It's always bioassays and trip reports. Well, people are fallible. (even moreso with a head full of acid.) Consider Shulgin's famous anecdote:
If Sasha Shulgin can be fooled, so can you. So "trust, but verify".While at sea, he suffered a serious thumb infection that required surgery. Handed a glass of juice by a nurse, he spotted some undissolved solids at the bottom of the glass, assumed it was a sedative and fell unconscious. It was actually sugar; the placebo effect had knocked him clean out.
This is like claiming the blotter art signifies different 'types' of LSD that produce different 'flavours' of trips. Or Cannabis sativa and Cannabis indica having differing effects (nowadays both are usually high THC low CBD cultivars). Or terpenes having major effects at all beyond smells. IME, all of these are old rumours that need to go away.
Otherwise I could mix my piss in your MG wine and claim that it makes a superactive hallucinogen from, I dunno, glucuronic acid or something, based upon solely my claim that "Well, I drank a 50/50 piss and MG wine mixture and had an intense and highly sexually charged trip that lasted 4 days." I could come up with a bunch of misread "evidence" papers too! But I don't because I'm trying to practice actual science and critical thinking.
Last edited:
- Joined
- Jul 22, 2018
- Messages
- 638
I never meant that at all. I'm sorry if I wasnt clear. I was saying any compound, (including fragile polar ones) can be extracted and isolated with the right process. Sometimes that process can be a lot simpler than what you might think.Easy as THC extraction? Not by a long shot
Understanding the physical properties of both the target and non target compounds as well as the different polarities of medias and solvent systems is critical.
Is it as easy as THC or CBD? No. Is it impossible? No, I think any trained process chemist could do it.
As far as all of this adduct business I think it's all nonsense/placebo. Thank you for taking your time to write such a detailed post refuting the claims. I agree with almost everything you said, I was too lazy to do all that research and refute it myself because I know it's not gonna matter. Tregar is gonna believe what he believes not matter what you tell him.
- Joined
- Jul 22, 2018
- Messages
- 638
"Since about 90% of the alkaloid pro-duced is found in the mycelium, the extraction was carried out by adding 10 ml of an aqueous solution of 4% tartaric acid to 5 ml of culture broth. The mixture was diluted to 40 ml with acetone and homogenized for 1 min. To 4 ml of the filtrate, 1 ml of a 1:2 mixture of borate buffer (0.4M) and NaOH (1N) was added, to obtain a pH value of about 10. This preparation was then extracted three times with 8 ml of CHCl3 (3ml+3ml+2ml), and the pooled extracts were diluted to10 ml with CHCl3."
This process looks like a slightly complicated acid base extraction with I assume a form of chromatography at the end to isolate ergotamine.
Sorry the formatting is so messed up, I would fix it, but I can only use a touch screen at the moment. Also that paper is from 1966, we may have well developed even easier methods by now.
This process looks like a slightly complicated acid base extraction with I assume a form of chromatography at the end to isolate ergotamine.
Sorry the formatting is so messed up, I would fix it, but I can only use a touch screen at the moment. Also that paper is from 1966, we may have well developed even easier methods by now.
Last edited by a moderator:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,483
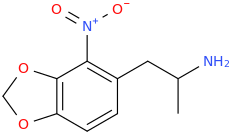
PEDRO
1-(3,4-methylenedioxy-2-nitrophenyl)-2-aminopropane
Vote 4 Pedro!!!
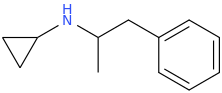
SPEED_FREAK
N-cyclopropyl-1-phenyl-2-aminopropane
"Ah...clean vapin propane!"--King Of The Hill.

LOW_BALLER_DICK_SMALLER
N-cyclopropyl-(4-methoxyphenyl)-2-aminopropane
High Roller!
Last edited:
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
As I noted in my previous post, it's definitely doable, but way more hassle than the usual. Nowhere did I make the claim that you were saying it was a trivial process, and I apologize if I came off that way. Most importantly you have to deal with the 100:10:0.1:0.045 ratio of volume:mycelium weight: product alkaloids:lysergic acid produced by plain C.paspali meaning you better have a badass reactor and a lot of solvents.
Anything with chromatography in it, I consider a non-starter except for cases of exceptional need. Even then, like you I prefer to do flash chromatography rather than columns, just to save sanity.
Anything with chromatography in it, I consider a non-starter except for cases of exceptional need. Even then, like you I prefer to do flash chromatography rather than columns, just to save sanity.
- Status
- Not open for further replies.














