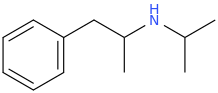
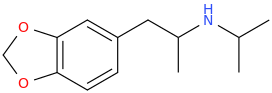
AlsoTapered
Bluelighter
I've written a post describing a ligand design process that is only 5 steps. Read it, learn it, understand it, use it then post it.
N&PD Moderators: Skorpio | someguyontheinternet
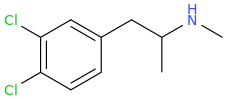

You say you are advanced chemist and than proceed to make statements that are similar to – coke and sugar have almost the same formula so therefore they are both addictive.https://www.shroomery.org/forums/showflat.php/Number/27850299/fpart/13
pic: I will post my bottle of isovaleraldehyde when I am back in town 2 weeks from now. But this is the aldehyde that will give you real LSD like effects and stimulation, 2 drops is all you need. Isovalderaldehyde has nearly the exact same chemical formula as the tail end of DMT or psilocin: CHO CH2 CH2 2(CH3), and nearly the same molecular weight 87g/mol as LSD's diethylamine at 73 g/mol. Both isovaleraldehyde from the Greece Eleusis kykeon peppermint and 3-aminopentane from Dr. Nichols have exactly 5 carbon groups and 11 hydrogen groups (once the aldehye attaches to one of the H already at the amide of LSA). Very similar to LSD's diethylamine which has 4 carbon groups and 11 hydrogen groups. See post above with the pics and study from LSD scientist Dr. Nichols as to why Rats respond to this number of carbons and hydrogens on the LSA amide as if it is real LSD.
This is ANCIENT LSD, it was hypothesized to form from ground claviceps paspali ergot which grows on paspalum distichum grass in the famous Rarian plane adjacent to Eleusis (same safe alkaloid profile as Mesoamerican morning glory high in LSA) that is mixed with a handful or large amount of fresh peppermint leaves for a while in wine. This could very well be the famous Kykeon psychedelic brew, formula kept secret for 2,000 years by the priests, able to easily feed over 300 hundred people (new initiates) like clockwork every Sept. LSA --> potent LSI, it is indeed possible and works extremely well.

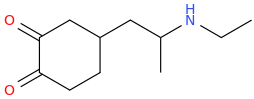

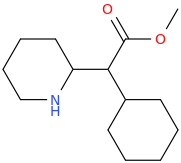

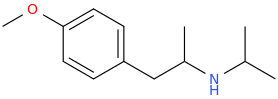



| Bioorganic & Medicinal Chemistry Letters vol. 5 iss. 6 pp.589—592 Stereoselective synthesis and evaluation of all stereoisomers of Z4349, a novel and selective μ-opioid analgesic DOI: 10.1016/0960-894x(95)00077-7 a a | M. Napoletano; Bella D. Delia; C. Fraire; G. Grancini; C. Masotto; S. Ricciardi; C. Zambon | 1995 March | English | ||
| ChemInform vol. 26 iss. 31 pp.no—no ChemInform Abstract: Stereoselective Synthesis and Evaluation of All Stereoisomers of Z4349, a Novel and Selective μ-Opioid Analgesic. DOI: 10.1002/chin.199531209 a a |
ID ↕ Time add. ↕ Title ↕ Series ↕ | Author(s) ↕ | Publisher ↕ | Year ↕ | Pages | Language | |
|---|---|---|---|---|---|---|
| ChemInform vol. 33 iss. 11 pp.no—no ChemInform Abstract: New μ-Opioid Receptor Agonists with Piperazine Moiety. DOI: 10.1002/chin.200211149 a a | Teruo Komoto; Tomomi Okada; Susumu Sato; Yasuhiro Niino; Tetsuo Oka; Takao Sakamoto | 2010 May 22 | ||||
| Journal of Computer-Aided Molecular Design vol. 7 iss. 5 pp.557—571 Computer-aided structure-affinity relationships in a set of piperazine and 3,8-diazabicyclo[3.2.1]octane derivatives binding to the μ-opioid receptor DOI: 10.1007/bf00124362 a a | Daniela Barlocco; Giorgio Cignarella; Giovanni Greco; Ettore Novellino | 1993 October | English | |||
| Journal of Theoretical and Computational Chemistry vol. 09 iss. supp01 pp.49—63 INTERACTION OF BRIDGED PIPERAZINE DERIVATIVES WITH THE μ-OPIOID RECEPTOR — A THEORETICAL MODEL DOI: 10.1142/S0219633610005566 a a |
It's not the number of atoms that matter, it's their connectivity and shape.See post above with the pics and study from LSD scientist Dr. Nichols as to why Rats respond to this number of carbons and hydrogens on the LSA amide as if it is real LSD
Isovaleraldehyde is definitely not related to peppermint. I used to work with the stuff. It smells like toe jam.Both isovaleraldehyde from the Greece Eleusis kykeon peppermint
The problem is, ergot doesn't make LSA. It makes ergolines (e.g. ergotamine), which need to be chemically processed to split out the lysergic acid. And morning glory / Hawaiian baby woodrose have a totally different alkaloid profile - I don't think they make any ergolines.This is ANCIENT LSD, it was hypothesized to form from ground claviceps paspali ergot which grows on paspalum distichum grass in the famous Rarian plane adjacent to Eleusis (same safe alkaloid profile as Mesoamerican morning glory high in LSA)