Helios.
Ex-Bluelighter
- Joined
- May 25, 2006
- Messages
- 1,843
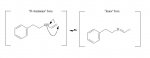
Some crackhead on the streets tried to sell me some 4-allyl-2,5-dimethoxypenethylamine the other day. Said it was the same as methamphetamine.
I doubt it, but don't see why it wouldn't be active, or why the DOAL form wouldn't be stronger than the 2CAL form.
I doubt it, but don't see why it wouldn't be active, or why the DOAL form wouldn't be stronger than the 2CAL form.