- Joined
- Jun 30, 2015
- Messages
- 1,596
Ive read about some weird lamp that can somehow stimulate brain waves..
Its supposed to give a dmt like effect by just sitting in the light in emits.
The placebo can be remarkably powerful
Ive read about some weird lamp that can somehow stimulate brain waves..
Its supposed to give a dmt like effect by just sitting in the light in emits.
Oh wow, that does sound....interesting. I'm not sure I would be in a hurry to be the first to self administer it, though. Can't predict much from 5-HO-DMT and the extended DiPT family, but they're all known for producing some subjectively dirty feeling bodyload.I seem to remember reading that 5-ho-dipt could be an interesting drug, but I can't remember where I read it...
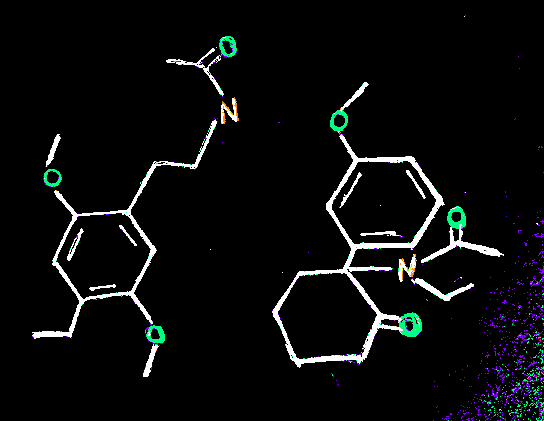
does putting an acyl group... anywhere on a molecule make it a prime suspect for being a prodrug?
for instance, if we were to put one on one of the methyls of dmt, would it be likely to hydrolize into dmt?
what about, say, the indole nitrogen of 5-meo-dmt?
edit
just wondering, i was thinking about this when i drew these

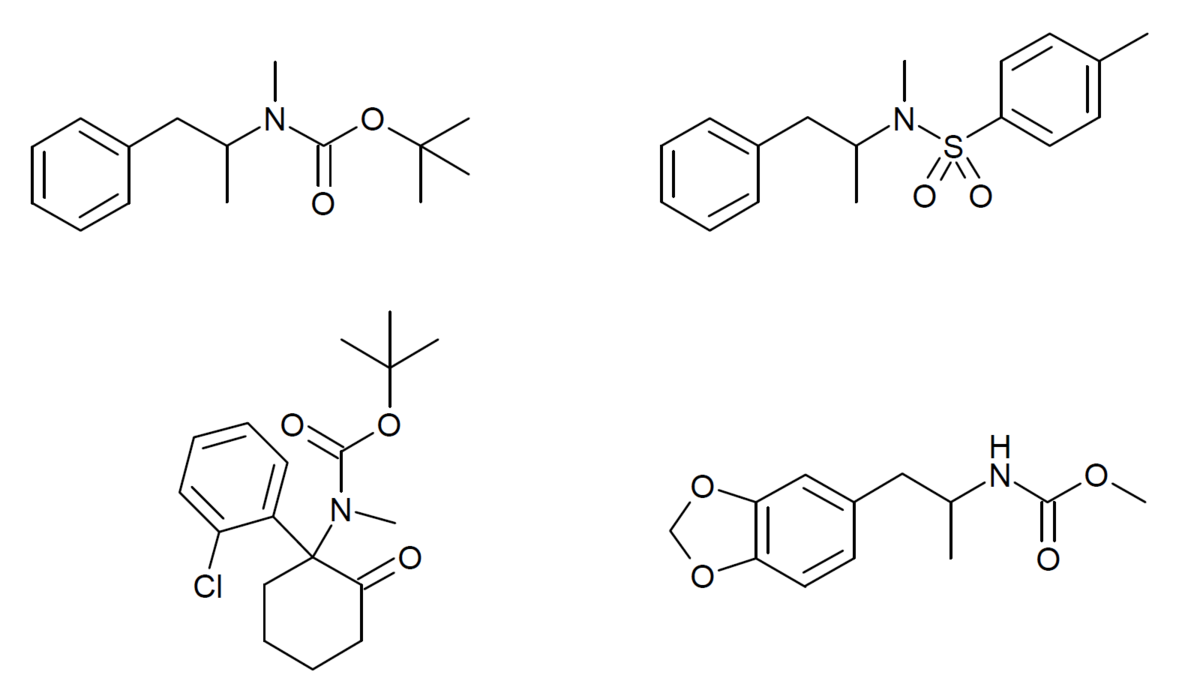
(please excuse my ignorance, i'm pretty wet behind the ears when it comes to chemistry)With the ACH example, tertiary amines get cleaved at the amine into potentially equivalent portions of the 2 resulting secondary amines (I don't think this has been confirmed yet, though it is known that an ACH with a diethylamine metabolizes into a bunch of regular PCE).
Oh no I should've specified this doesn't happen with cyclic amines for whatever reason(please excuse my ignorance, i'm pretty wet behind the ears when it comes to chemistry)
does this also mean cyclic amine substitutions (like PCP) get metabolized into chain tertiary amines, and continue along the line into PCE, PCM, PCA etc.?
does this mean the ethyl/ acyl-amine would be the same as having the diethyl amine? (edit, i think i get what you mean... the double bonded oxygen means no fully saturated carbon, which means no binding to NMDAr, until it's metabolized?)
if it metabolizes into MXE, why haven't we seen any 3-methoxy-2'-oxo-PCdiethyl delightfulness? it would seem to be a shoe-in for popularity, if i understand what you're saying.
Woah that's pretty neat. If you have a source on the metabolism I'd love to read further, I searched N,N-diethyl-1-phenylcyclohexylamine ("PCDEA")though it is known that an ACH with a diethylamine metabolizes into a bunch of regular PCE
