-
MDMA &
Empathogenic
Drugs
Welcome Guest! -
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is wrong with the MDMA available today?
- Thread starter Le Junk
- Start date
- Status
- Not open for further replies.
babooon87
Bluelighter
- Joined
- Dec 12, 2008
- Messages
- 185
So yeah, sample 1, brown/transparent (how is that even possible ?) rocks weighted at 78 mg is definitely Mehhh. Still 4 samples to go throught. There is one that he seemed pretty greedy and defensive with that looked good and is probably the magic stuff. His attitude was really off about that sample....
Not a whole alot of information out there expect that study looking at the effects on the receptors. Not something that would attract funding aswell. Maybe try find a way to contact the papers author with questions?
See your not thinking... WHAT IS THIS ABSTRACT? Kinda goes above my head but a simple google search of carbonyl serotoin yielded this... I can dissect pieces... pharmacology isnt my specialty clandestine synthesis is sorry...
In vivo binding properties of [carbonyl-11C]WAY-100635: effect of endogenous serotonin.
[Carbonyl-(11)C]WAY-100635 has been reported to be a useful ligand for the investigation of 5-HT(1A) receptor imaging in vivo. However, the cellular distribution and the influence of endogenous serotonin (5-HT) on in vivo binding have not been fully examined. In this study, we investigated the effect of 5,7-dihydroxytryptamine-produced destruction of 5-HT neurons, reserpine-induced 5-HT depletion, and fenfluramine-induced 5-HT increase on [carbonyl-(11)C]WAY-100635 binding in vivo. There was no significant change in the uptake of [carbonyl-(11)C]WAY-100635 in the slice of 5-HT denervated rat brain except in the raphe nucleus, where 5-HT cell bodies exist. There was no obvious effect of enhanced 5-HT release by fenfluramine or decreased release by reserpine on [carbonyl-(11)C]WAY-100635 binding in the dissected brain region. No significant effect was observed in the time course of [carbonyl-(11)C]WAY-100635 in the hippocampus and frontal cortex measured by PET. These results indicated that the in vivo binding of [carbonyl-(11)C]WAY-100635 in the hippocampus and cerebral cortex mainly reflects postsynaptic 5-HT(1A) receptor binding, and that this binding is not sensitive to endogenous 5-HT.
WAY-100635 is a piperazine drug you know similar too
Recreational Drugs
- 4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP)
- 1-Benzylpiperazine (BZP)
- 2,3-Dichlorophenylpiperazine (DCPP)
- 1,4-Dibenzylpiperazine (DBZP)
- 4-Methyl-1-benzylpiperazine (MBZP)
- 3-Chlorophenylpiperazine (mCPP)
WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor.
So this is a carbonyl-11C BZP analog similar to our MDMA carbonyl-11 or 12C analog?
Last edited:
indigoaura
Bluelighter
- Joined
- Jan 4, 2009
- Messages
- 1,707
@vash445, you posted that, "MDMA has ten times more affinity for uptake at serotonin transporters compared to dopamine and norepinephrine transporters and consequently has mainly serotonergic effects.[95]:1080 "
Earlier, in the report, it implied that the impurities had a greater effect on serotonin receptor systems than dopamine (based on my limited interpretation).
"...suppressed MDMA-induced release mediated by the SERT more potently than NET-mediated release, whereas MDMA-induced release mediated by the DAT was only weakly inhibited in concentrations up to 100 M (Fig. 8C). " (Link)
Theoretically, this could explain why the mehDMA still has some effects like jaw shaking etc, but lacks the serotonergic effects like love and empathy. Since it feels "weak" but similar to MDMA in some ways, people take much higher doses than they should, which could contribute to the physically difficult come-downs. It would also explain why there is no typical "suicide Tuesday," because there is no serotonin crash. Of course, the impurities would also be contributing to the physically rough come-downs.
Earlier, in the report, it implied that the impurities had a greater effect on serotonin receptor systems than dopamine (based on my limited interpretation).
"...suppressed MDMA-induced release mediated by the SERT more potently than NET-mediated release, whereas MDMA-induced release mediated by the DAT was only weakly inhibited in concentrations up to 100 M (Fig. 8C). " (Link)
Theoretically, this could explain why the mehDMA still has some effects like jaw shaking etc, but lacks the serotonergic effects like love and empathy. Since it feels "weak" but similar to MDMA in some ways, people take much higher doses than they should, which could contribute to the physically difficult come-downs. It would also explain why there is no typical "suicide Tuesday," because there is no serotonin crash. Of course, the impurities would also be contributing to the physically rough come-downs.
indigoaura
Bluelighter
- Joined
- Jan 4, 2009
- Messages
- 1,707
Also, seems like our working thesis is evolving. Currently, appears that: improper synthesis methods are resulting in impurities and/or isomers that reduce the effects of MDMA at the receptor level, but are challenging to identify in typical GCMS testing due to similar molecular weights.
Does that accurately summarize where we are currently? I want to keep Drugs Data in the loop. They have had some issues in their offices and have not been able to move forward on their end yet, but are still planning to. If we have reports and additional information, I can forward that info to them and it may help them know where to start with updating their procedures to be able to identify these contaminants.
Does that accurately summarize where we are currently? I want to keep Drugs Data in the loop. They have had some issues in their offices and have not been able to move forward on their end yet, but are still planning to. If we have reports and additional information, I can forward that info to them and it may help them know where to start with updating their procedures to be able to identify these contaminants.
yes that is about all we know.Also, seems like our working thesis is evolving. Currently, appears that: improper synthesis methods are resulting in impurities and/or isomers that reduce the effects of MDMA at the receptor level, but are challenging to identify in typical GCMS testing due to similar molecular weights.
Does that accurately summarize where we are currently? I want to keep Drugs Data in the loop. They have had some issues in their offices and have not been able to move forward on their end yet, but are still planning to. If we have reports and additional information, I can forward that info to them and it may help them know where to start with updating their procedures to be able to identify these contaminants.
the images below have all the peaks assigned. this will be one of 3 post,
first the 1H NMR
The NMR is very similar to what would be expected for MDMA, but there are a couple of weird things. The broad resonance at 9ppm is most likely to be protonated amine not carbonyl, as there is no carbonyl carbon in the 13C spectra. I will review the 13c results in the next post.
The resonances around 2.5 ppm are being covered by the DMSO solvent. An expansion of the region at 2.5ppm would make it possible to see with a bit more detail what is going on. however on the left there is one of the expected benzylic position protons, there is an unidentified peak X to the right upfield of DMSO at 2.497 ppm this could be amine but it is strange that there are two distinct amine resonances as peak at 2.072 could be N-H too.
The 9ppm peak is consistent with some amine hydrochlorides in DMSO but there is no info on mdma.hcl in dmso.
3.5 is water it should be 3.3 in DMSO but it can move around
The aromatic region is as expected, it is methylenedioxy and 3,4 pattern.

first the 1H NMR
The NMR is very similar to what would be expected for MDMA, but there are a couple of weird things. The broad resonance at 9ppm is most likely to be protonated amine not carbonyl, as there is no carbonyl carbon in the 13C spectra. I will review the 13c results in the next post.
The resonances around 2.5 ppm are being covered by the DMSO solvent. An expansion of the region at 2.5ppm would make it possible to see with a bit more detail what is going on. however on the left there is one of the expected benzylic position protons, there is an unidentified peak X to the right upfield of DMSO at 2.497 ppm this could be amine but it is strange that there are two distinct amine resonances as peak at 2.072 could be N-H too.
The 9ppm peak is consistent with some amine hydrochlorides in DMSO but there is no info on mdma.hcl in dmso.
3.5 is water it should be 3.3 in DMSO but it can move around
The aromatic region is as expected, it is methylenedioxy and 3,4 pattern.

next 13C
There is not much unexpected here, it is consistent with MDMA and there is only one carbon that shouldn't be there.

Note there are no resonances left of 150ppm (the 3 and 4 position aromatic carbons) no carbonyls no amides no esters no carboxylic acids no carbonyls.
and on to the next post wtf does this all mean
There is not much unexpected here, it is consistent with MDMA and there is only one carbon that shouldn't be there.

Note there are no resonances left of 150ppm (the 3 and 4 position aromatic carbons) no carbonyls no amides no esters no carboxylic acids no carbonyls.
and on to the next post wtf does this all mean
Last edited:
indigoaura
Bluelighter
- Joined
- Jan 4, 2009
- Messages
- 1,707
Looking forward to the wtf does this all mean post. :D
next 13C
There is not much unexpected here, it is consistent with MDMA and there is only one carbon that shouldn't be there.
View attachment 17292
Note there are no resonances left of 150ppm (the 3 and 4 position aromatic carbons) no carbonyls no amides no esters no carboxylic acids no carbonyls.
and on to the next post wtf does this all mean
So I took a better carbon today. That mystery peak is an impurity and is likely part of the same molecule that the new mini peak at 207 is part of......And we see that a peak at 207 .....An aldehyde or ketone!!!!! Yeaaaa!!!!!Your friends analysis is all correct but still doesn't tell us what's happening. He is just as confused as I am. It has carbonyl impurities, It's structure seems very close to what it should look like in nmr but not quite...

Ideas??
On the face of it this NMR is MDMA. the supposed potency and effects of this are not matching MDMA. Or that is what everyone thinks.
if this is not MDMA it a mixture of something that has practically the same NMR spectra as MDMA both 1H and 13C which means it has to contain the same carbon backbone and the same structural features, it cannot be an isomer because there are no isomers of MDMA which have the same mass and the same NMR spectrum.
The 2D NMR is interesting and probably holds some useful clues.
one candidate for the problem that would fit with the data so far is the a dimer,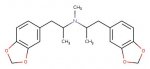
this has practically the same NMR as MDMA, with NMR using D2O as the solvent, it would be almost impossible to tell this apart from MDMA. in general if this was contaminating MDMA it would be very very hard to spot indeed
GC MS would only see it if the chromatography run was long enough and the mass spec scanning high enough.
This can be formed if the stoichimetry in reductive amination is wrong, too much ketone not a big enough excess of methylamine or methylamine equivalent. It doesn't matter what reductive amination is done, leuckart, Al/Hg, cat H2, Borohydride if there is not a large enough excess of methylamine the dimer is going to form. The MDA version fo this dimer can also form from reduction of methylenedioxyphenylnitro propene (nitrostyrene).
this is most likely a serotonin reuptake inhibitor but it is not known whether it reduces the effects of mdma in humans.
Vash if you want to get your NMR guy to run it again in D2O and then D2O with a drop of water that would probably give very useful information.
somebody else with Meh needs to get it NMR analysed to see if it is similar to this stuff. then good stuff needs to be compared to Meh with NMR and that would give the definitive proof that there is something to this.
On the face of it this NMR is MDMA. the supposed potency and effects of this are not matching MDMA. Or that is what everyone thinks.
if this is not MDMA it a mixture of something that has practically the same NMR spectra as MDMA both 1H and 13C which means it has to contain the same carbon backbone and the same structural features, it cannot be an isomer because there are no isomers of MDMA which have the same mass and the same NMR spectrum.
The 2D NMR is interesting and probably holds some useful clues.
one candidate for the problem that would fit with the data so far is the a dimer,

this has practically the same NMR as MDMA, with NMR using D2O as the solvent, it would be almost impossible to tell this apart from MDMA. in general if this was contaminating MDMA it would be very very hard to spot indeed
GC MS would only see it if the chromatography run was long enough and the mass spec scanning high enough.
This can be formed if the stoichimetry in reductive amination is wrong, too much ketone not a big enough excess of methylamine or methylamine equivalent. It doesn't matter what reductive amination is done, leuckart, Al/Hg, cat H2, Borohydride if there is not a large enough excess of methylamine the dimer is going to form. The MDA version fo this dimer can also form from reduction of methylenedioxyphenylnitro propene (nitrostyrene).
this is most likely a serotonin reuptake inhibitor but it is not known whether it reduces the effects of mdma in humans.
Vash if you want to get your NMR guy to run it again in D2O and then D2O with a drop of water that would probably give very useful information.
somebody else with Meh needs to get it NMR analysed to see if it is similar to this stuff. then good stuff needs to be compared to Meh with NMR and that would give the definitive proof that there is something to this.
Last edited:
Looking forward to the wtf does this all mean post. :D
The answer is Both of them really don't know it seems XD
Ideas??
On the face of it this NMR is MDMA. the supposed potency and effects of this are not matching MDMA. Or that is what everyone thinks.
if this is not MDMA it a mixture of something that has practically the same NMR spectra as MDMA and MDMA, both 1H and 13C which means it has to contain the same carbon backbone and the same structural features, it cannot be an isomer because there are no isomers of MDMA which have the same mass and the same NMR spectrum.
The 2D NMR is interesting and probably holds some useful clues.
one candidate for the problem that would fit with the data so far is the a dimer, View attachment 17296
this has practically the same NMR as MDMA, with NMR using D2O as the solvent, it would be almost impossible to tell this apart from MDMA. in general if this was contaminating MDMA it would be very very hard to spot indeed
GC MS would only see it if the chromatography run was long enough and the mass spec scanning high enough.
This can be formed if the stoichimetry in reductive amination is wrong, too much ketone not a big enough excess of methylamine or methylamine equivalent. It doesn't matter what reductive amination is done, leuckardt, Al HG, cat H2, Borohydride if there is not a large enough excess of methylamine the dimer is going to form. The MDA version fo this dimer can also form from reduction of methylenedioxyphenylnitro propene (nitrostyrene).
this is most likely a serotonin reuptake inhibitor but it is not known whether it reduces the effects of mdma in humans.
Vash if you want to get your NMR guy to run it again in D2O and then D2O with a drop of water that would probably give very useful information.
somebody else with Meh needs to get it NMR analysed to see if it is similar to this stuff. then good stuff needs to be compared to Meh with NMR and that would give the definitive proof that there is something to this.
@indigoaura has donated 2 samples of MEH. I have procured and tested a batch of magic active at 90-110mg soo... this is after the "failed" halloween roll
NMR guy is out of of this meh but I still have some I just gonna send it all over.
I personally think that this material is incompetent synthesis done by clueless idiots. mix clueless idiots with clandestine chemistry and god only knows what you create. Combine shoddy synthesis with no proper purifcation and the result is predictably junk, but junk that people buy and consume anyway because thanks to the war on drugs there is no legitimate alternative.
share the new 13C please.
can another 1H in D2O be run I think the wierd data is consistent with dimer at the moment not formyl
Give me a day or 2 i'll get it up here.
opposable-thumbs
Greenlighter
- Joined
- Nov 17, 2019
- Messages
- 18
Read all 162 pages and had to sign up to say thank you to everyone trying to figure out this mystery.
My first time with MDMA was in my mid-30s in 2015. Managed to get my hands on some (sourced in-person via connection on the west coast of USA) for my wife and to try, and it was truly the definition of MagicDMA.
Super loved up, massive pupils, open, euphoric, rubbing on each other all night, lasted 6 hours, hard to sleep, next day afterglow. My wife usually badly chewed the inside of her cheeks even with taking measures to prevent it. Tan crystal, smelled strongly like safrole, tasted terrible, went purple to black with Marquis.
Had enough from that batch to last a year and a half for both of us rolling every 3-4 months.
Everything I've been able to get ahold of since then (DNM sourced, personally tested with Marquis, Mandelin, Mecke, Simons reagents) has been meh.
Usually white or very light greyish/tan crystals that go immediately black with Marquis, no hint of purple. Faint or no smell, taste is always more salty than bitter.
Come up with no euphoria, very little if any pupil dilation, open but much more introspective, lasts 3-4 hours, tired before it even wears off, wife doesn't chew her cheeks.
I'm starting to believe that the original batch of magic I had was a west coast or possibly even Canadian made product.
My source at the time was very well connected in those circles.
All the meh has either been sourced from European or Canadian (probably reselling European product) DNM vendors.
Very interested in seeing how this all turns out.
My first time with MDMA was in my mid-30s in 2015. Managed to get my hands on some (sourced in-person via connection on the west coast of USA) for my wife and to try, and it was truly the definition of MagicDMA.
Super loved up, massive pupils, open, euphoric, rubbing on each other all night, lasted 6 hours, hard to sleep, next day afterglow. My wife usually badly chewed the inside of her cheeks even with taking measures to prevent it. Tan crystal, smelled strongly like safrole, tasted terrible, went purple to black with Marquis.
Had enough from that batch to last a year and a half for both of us rolling every 3-4 months.
Everything I've been able to get ahold of since then (DNM sourced, personally tested with Marquis, Mandelin, Mecke, Simons reagents) has been meh.
Usually white or very light greyish/tan crystals that go immediately black with Marquis, no hint of purple. Faint or no smell, taste is always more salty than bitter.
Come up with no euphoria, very little if any pupil dilation, open but much more introspective, lasts 3-4 hours, tired before it even wears off, wife doesn't chew her cheeks.
I'm starting to believe that the original batch of magic I had was a west coast or possibly even Canadian made product.
My source at the time was very well connected in those circles.
All the meh has either been sourced from European or Canadian (probably reselling European product) DNM vendors.
Very interested in seeing how this all turns out.
^^^Yea good MDMA has zero salty taste. I’ve seen lots of, presumably kids, on Reddit talking about MDMA is supposed to taste salty and I just shake my head..
Thanks for stopping in to share your experience and appreciate you taking the time to read it all
-GC
Thanks for stopping in to share your experience and appreciate you taking the time to read it all
-GC
The dimer theory is a good one..
If we are to be blaming large scale productions for being the problem it’d make sense that they try to cut costs in any way possible. One such way would be to minimize methylamine usage since its not an easy precursor to obtain in the quantities they need.
If they can make a product which tests legit in the lab but requires less methylamine, even if it feels “less good” they’ll still likely go for it because of the money.
The problem then becomes how to separate the dimer easily at home.
-GC
If we are to be blaming large scale productions for being the problem it’d make sense that they try to cut costs in any way possible. One such way would be to minimize methylamine usage since its not an easy precursor to obtain in the quantities they need.
If they can make a product which tests legit in the lab but requires less methylamine, even if it feels “less good” they’ll still likely go for it because of the money.
The problem then becomes how to separate the dimer easily at home.
-GC
- Status
- Not open for further replies.




