So if I'm understanding correctly, not enough methylamine in the reaction results in an excess of MDP2P (or other precursor?) as it did not have methylamine to react (?) with. Without enough methylamine, MDP2P is left unreacted and MDMA-dimer is created instead of MDMA?
I'm confused why anything is created at all once the methylamine is used up and the MDP2P is simply left untouched, both ingredients are out of the equation. Is MDMA-dimer the result of everything else minus those two?
Just trying to convert all this is to sensical laymans terms
Still, out of ED's 8000 entries MDP2P/Pol is not terribly common at all. Would MDP2P/other precursor excess always be present if Dimer was created? Or could that be removed after the fact (make a batch without enough methylamine, wash out the MDP2P, and voila you have what passes as straight MDMA but has MDMA-dimer hiding?)
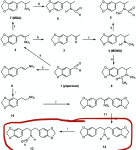
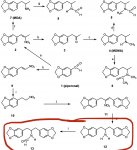
the reaction reductive amination is in essence 2 steps, the reversible reaction of a carbonyl compound (MDP2P in this case) with an amine, Methylamine in this case to give an
imine which is then reduced to an amine by the reducing agent Al(Hg) NaBH4 Pt H2 or whatever. If there is not enough methylamine then some of the MDP2P will be unreacted and not converted to
imine, if the reduction step is not performed correctly then some of the
imine will remain, which will hydrolyze to amine and starting MDP2P when the reaction is worked up. if there is not enough methylamine there will also be reduction of the MDP2P to MDP2Pol.
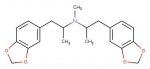
The problem is that the product MDMA is an amine too, just like methylamine, and this can react with the starting carbonyl to make an imine like compound which is then reduced to the dimer. The strategy of using excess methylamine is to make it much more likely that the carbonyl collides and reacts with methylamine to make MDMA, rather than the product amine MDMA (making the dimer). This problem is more likely if the reaction is run too concentrated and a couple of other things are important but I am not going to go into those details.
So whilst MDP2P should not be in the product, and to be seen by GCMS software it needs to be greater than 1% of the largest peak area. it is conceivable that a product could contain MDP2P but not significant dimer because there was enough methylamine but the
imine was not reduced, the second part of the reductive amination was rushed or screwed up and so the unreduced imine hydrolyzed back to MDP2P and methylamine. It is however a clear sign of a screwed up synthesis.
Likewise no MDP2P does not mean the reaction was run right, because the MDP2P is nonpolar if the MDMA hydrochloride was washed with a solvent then the MDP2P would be washed out. the dimer because it is also an amine hydrochloride would not be washed out. Vacuum distillation of the freebase or proper recrystalization of the hydrochloride salt would remove the dimer if it was present almost completely.
There are a whole load of other factors that a real process chemist would nail down, pH, temperature, reagent proportions, concentration, stirring, work up, starting material purity. All of these things would be varied in tests and the side reactions identified and those that were found to be critical would be tightly controlled but clandestine synthesis is cheap and nasty and there is no control of the reaction and no control of the product purity.
Methylamine certainly appears difficult for clandestines to source in large quantities, it also really stinks and excess stinky methylamine will be in the product and the various liquids, and so there is going to be a huge temptation for clandestines to reduce to the minimum the methylamine (or methylamine surrogate like nitromethane) they use. There must also be a temptation to rush the synthesis, because they don't want to get caught. so half run reactions and unconverted starting material is quite likely, then add total lack of purification of the product and see the crap that is getting into the final material.
The dimer does go through GCMS but it comes out very very late. I don't thing EC or the others are looking for it and if it is under 1% their software would ignore it anyway.
I will comment on the 13C spectra later.
good work people!