-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dresden's Chemical Fluff Thread (Name-A-Molecule)
- Thread starter Dresden
- Start date
I havnt, interesting albeit a report (somewhere in bluelight) said it is inactive also, which idk why.
Interestingly, the MPH without nitrogen, just a cyclohexane, is reported to be active, but weak.
Say interreting because it is a DARI without an amine. Similary the cocaine with C replacing N is also somewhat (very weakly) active too.
Try overlaying MPH to coke, and see where links where (in 3D ofc)
Interestingly, the MPH without nitrogen, just a cyclohexane, is reported to be active, but weak.
Say interreting because it is a DARI without an amine. Similary the cocaine with C replacing N is also somewhat (very weakly) active too.
Try overlaying MPH to coke, and see where links where (in 3D ofc)
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
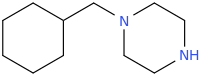
Rather than benzylmorpholine, IMO benzhydrylmorpholine would have much more chance of being active, with the morpholine nitrogen being one carbon away from the connection of the morpholine ring carbon to the benzhydryl bridging carbon. It'd be pretty close to desoxypipradrol in structure, Perhaps shorter acting due to the ether bridge in the morpholine ring.
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
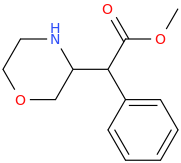
ED(WARD)
Predicted functional stim, Take Two.
Say It Ain't So. Your Drug Is A Heartbreaker--Weezer.
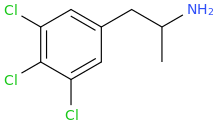
SHIVA
3,4,5-trichloroamphetamine.hcl
Fraid So.
It Is Absolutely Bizarre.
But I'm Also
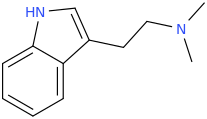
The Holy Spirit
And
AMP
Those Who Persecute The Holy Ghost Will Not Be Forgiven
You Have Been Warned.
Last edited by a moderator:
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
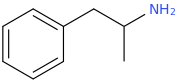
Carbomethoxy esters *may* be bioisosteric with phenyl groups, but they also add a wonderful fruity highish flavor and keep the molecule from lasting an eternity.
And Even With All My Logic And My Theory, I Add A Motherfucker So You Ignant Niggas Hear Me
^--not direct at you or anyone in particular for that matter, Pomzazed
Bioassaying
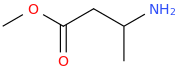
would be a sure way to test your hypothesis, yes?
The Best Thing About Being A Titan Is That, In The Final Analysis, We Are Completely Unstoppable.--Prometheus. August 30th, 2018 AD.
And Even With All My Logic And My Theory, I Add A Motherfucker So You Ignant Niggas Hear Me
^--not direct at you or anyone in particular for that matter, Pomzazed
Bioassaying

would be a sure way to test your hypothesis, yes?
The Best Thing About Being A Titan Is That, In The Final Analysis, We Are Completely Unstoppable.--Prometheus. August 30th, 2018 AD.
Last edited by a moderator:
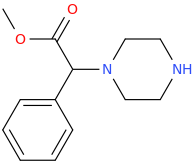
Lol you are so fast to grab that idea. I chuckle alot at above molecule, interesting!
- Well you are correct about *may*; as each isostere is applicable to some class of cmpd only, not one rule fit all and repleaceable for all.
- methanolysis of 2-methylpyrolidone to get that pdt huh? I guess the reversible reaction is true and favoured even in storage, as that NH2 is very nucleophilic and 5-mem ring is such favoured kinetically and somewhat favoured thermodynamically
Edit:
Stealing Dresden's idea...
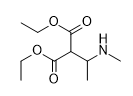
HUZZAH
- Well you are correct about *may*; as each isostere is applicable to some class of cmpd only, not one rule fit all and repleaceable for all.
- methanolysis of 2-methylpyrolidone to get that pdt huh? I guess the reversible reaction is true and favoured even in storage, as that NH2 is very nucleophilic and 5-mem ring is such favoured kinetically and somewhat favoured thermodynamically
Edit:
Stealing Dresden's idea...

HUZZAH
Last edited:
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
Haha, that's funny. Huzzah!
The 4-chloroamphetamines:
4-chloroamphetamine
HILLARY RODHAM CLINTON
4-chloromethamphetamine
JAY "PEPSI" MARTIN
N-ethyl-4-chloroamphetamine
SWEET "JANE" MARTIN
Werd to mutha, I really don't care if these give you brain damage or not. If you don't want your brain, which has intrinsic plasticity, to evolve (or 'change') over time, then don't take psychotropic medications at all, including prescription antidepressants for example!
HILLARY showed up in pressed "Diablo XXX" pills and was compared with the very best of MDMA rolls!
The 4-chloroamphetamines:
4-chloroamphetamine
HILLARY RODHAM CLINTON
4-chloromethamphetamine
JAY "PEPSI" MARTIN
N-ethyl-4-chloroamphetamine
SWEET "JANE" MARTIN
Werd to mutha, I really don't care if these give you brain damage or not. If you don't want your brain, which has intrinsic plasticity, to evolve (or 'change') over time, then don't take psychotropic medications at all, including prescription antidepressants for example!
HILLARY showed up in pressed "Diablo XXX" pills and was compared with the very best of MDMA rolls!
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
You've ALREADY posted the whole gamut of ring-trihalogenated versions of various amphetamines. What is the point in posting the same things multiple times?
Oh, and for a whole load of this crap (admittedly you seem to actually be putting effort into coming up with things with a reason, or at least, intended reasoning, behind them now, which is good), there is no logic or wisdom behind it. There is a lot of just drawing pictures, giving them some ridiculous name and a load of utter bollocks about deities. That is pointless in the extreme, serving nobody, not us, and not you either. Thats just garbage, all that fucking horse-shit about deities.) And it certainly ain't current IUPAC, unless they have DRASTICALLY changed their system within the time this post first started.
And the para-haloamphetamines, dresden, with the exclusion of fluorine, aren't just bad for people, they are blatant neurotoxins. And they are used via microinjection into brain regions in animal studies, to cause permanent lesions of the serotonergic pathways targeted. Not just nasty, but outright poisons.
And whoever released PCA on the RC market needs to have a broken chair stuffed up their arse. Diagonally.
PCA at one point was IIRC released by big pharma as an antidepressant candidate, until it was found out the non-fluoro para-halogenated amphetamines (with no other substitution, E.g the likes of 2C-C. DOC, DOB, DOI etc.) were dangerous and long-term neurotoxins. They would probably also cause severe, lasting depression/crashes given the para-halo (excl. F) amphetamines cause a massive drop in serotonin production. Downright unpleasant little fuckers, and none of them should ever see the light of day outside of a lab (and IMO I find it pretty fucking disgusting they ever see the light of day *IN* one, at least in animal studies, its sick and twisted. Should stay as reference standards and possibly precursors) but otherwise, thats some sick, sick shit in my book. Yes, I realise why animal research is done, doesn't mean I have to like or agree with it)
Also, be aware of the existence of what are known as pseudohalogens, bioisosteres and often chemically resembling halogens, such as cyano, nitro groups, thiocyanato, pentafluorosulfanyl amongst others. Azide would be another, but you don't ever want an azido group on a drug of recreational intent, azido groups are unstable and make invariably for covalent, irreversibly binding ligands, splitting off N2 to form an amine. Can be useful in syntheses to produce amines (with care for azides are toxic, inhibiting the electron transport chain, like cyanide anion does, and those used in synthesis, usually sodium azide, NaN3, or other alkali metal azides, are somewhat explosive, and sensitive in the solid state, although they deflagrate rather than detonate (I.e burn fast, lots of hot expanding gases produced, they use sodium azide in car airbag charges, along with additives to mop up the sodium metal that is also liberated during a car crash when the charge goes off to inflate the airbag), going off as gunpowder does, rather than as a high explosive (I.e brisant, detonating not due to a fuel-oxidizer mix,the molecule of high-explosive flying apart releasing the large amounts of chemically stored energy, detonating, at a far higher speed than a deflagrating fuel-oxidizer 'low explosive' like gunpowder or flash powders.)
So things like para-nitroamphetamine, para-cyanoamphetamine would almost certainly be serotonergic neurotoxins where placed in the para-position of an otherwise unsubstituted (on the ring) amphetamine. Possibly any highly electron-withdrawing functional group para to the alkylamino sidechain if the phenyl ring bears otherwise only hydrogen; I certainly wouldn't be taking any of them,
Which isn't to say you want either to happen. This holds true only for alkali metal and quite likely alkaline earth metal azides. Heavy metal azide salts like lead azide are sensitive, impact-sensitive, friction-sensitive etc. high explosives, very sensitive, known as primary explosives, lead azide having been used like mercury fulminate has, in the primers of gun cartridges that are located in the back of the round and which are impacted by the firing pin or hammer of the firearm, heavy metal azides, as well as IIRC transition metal azides are sensitive primary high explosives. As well as being toxic of course.
Hydrazoic acid itself is unstable. Not so unstable as not to exist, but its a sensitive explosive, and in all but dilute solutions it is highly explosive. I kinda think of it as 'hydrogen cyanide/hydrocyanic acid that explodes too'. Also more difficult to treat cases of poisoning than with cyanides. In the case of cyanide poisoning it is possible to treat it, first with an inhaled alkyl nitrite, to keep you alive long enough to administer the rest of the antidote, consisting of intravenous sodium nitrite (not nitrate, nitrIte) then thiosulfate, the nitrites convert haemoglobin to methhaemoglobin, disrupting the ability of cyanide anion to bind to haemoglobin's iron center (CN- has great siderophilic tendencies, a huge affinity for iron depending on the oxidation state of the iron), and then the cyanide is mopped up by thiosulfate, which is converted to thiocyanate and excreted. vitamin B12, as the hydroxocobalamin form is also used (B12 has several forms, including hydroxocobalamin and cyanocobalamin).
The thiocyanate, unlike cyanide, can be dialyzed, too, helping the body to get rid of it, although it is nowhere NEAR as toxic as cyanide.
Granted in cases of cyanide poisoning, treatment has to be extremely rapid, given how rapidly cyanide can poison someone fatally, but in the case of poisoning by azide, it can't be treated with a cyanokit,despite its also borking up the electron transport chain and inhibiting cellular respiration, azide poisoning is far more difficult to treat.
Talking of bioisosteres Pomzazed, can you perhaps point me towards any books on the subject, either that you know of download links for, or that I can grab with sci-hub or LibGen?
I'm very interested indeed on this subject, what can pose as a bioisostere for what, and especially with your mentioning that some groups only act as bioisoteres for other groups within certain specific contexts. I'd like to learn more about both, both bioisosteres in general, and what the limits are, where something one would think to be a bioisostere for a functional group, would in fact, in a specific context, not in fact, do so.
Oh, and for a whole load of this crap (admittedly you seem to actually be putting effort into coming up with things with a reason, or at least, intended reasoning, behind them now, which is good), there is no logic or wisdom behind it. There is a lot of just drawing pictures, giving them some ridiculous name and a load of utter bollocks about deities. That is pointless in the extreme, serving nobody, not us, and not you either. Thats just garbage, all that fucking horse-shit about deities.) And it certainly ain't current IUPAC, unless they have DRASTICALLY changed their system within the time this post first started.
And the para-haloamphetamines, dresden, with the exclusion of fluorine, aren't just bad for people, they are blatant neurotoxins. And they are used via microinjection into brain regions in animal studies, to cause permanent lesions of the serotonergic pathways targeted. Not just nasty, but outright poisons.
And whoever released PCA on the RC market needs to have a broken chair stuffed up their arse. Diagonally.
PCA at one point was IIRC released by big pharma as an antidepressant candidate, until it was found out the non-fluoro para-halogenated amphetamines (with no other substitution, E.g the likes of 2C-C. DOC, DOB, DOI etc.) were dangerous and long-term neurotoxins. They would probably also cause severe, lasting depression/crashes given the para-halo (excl. F) amphetamines cause a massive drop in serotonin production. Downright unpleasant little fuckers, and none of them should ever see the light of day outside of a lab (and IMO I find it pretty fucking disgusting they ever see the light of day *IN* one, at least in animal studies, its sick and twisted. Should stay as reference standards and possibly precursors) but otherwise, thats some sick, sick shit in my book. Yes, I realise why animal research is done, doesn't mean I have to like or agree with it)
Also, be aware of the existence of what are known as pseudohalogens, bioisosteres and often chemically resembling halogens, such as cyano, nitro groups, thiocyanato, pentafluorosulfanyl amongst others. Azide would be another, but you don't ever want an azido group on a drug of recreational intent, azido groups are unstable and make invariably for covalent, irreversibly binding ligands, splitting off N2 to form an amine. Can be useful in syntheses to produce amines (with care for azides are toxic, inhibiting the electron transport chain, like cyanide anion does, and those used in synthesis, usually sodium azide, NaN3, or other alkali metal azides, are somewhat explosive, and sensitive in the solid state, although they deflagrate rather than detonate (I.e burn fast, lots of hot expanding gases produced, they use sodium azide in car airbag charges, along with additives to mop up the sodium metal that is also liberated during a car crash when the charge goes off to inflate the airbag), going off as gunpowder does, rather than as a high explosive (I.e brisant, detonating not due to a fuel-oxidizer mix,the molecule of high-explosive flying apart releasing the large amounts of chemically stored energy, detonating, at a far higher speed than a deflagrating fuel-oxidizer 'low explosive' like gunpowder or flash powders.)
So things like para-nitroamphetamine, para-cyanoamphetamine would almost certainly be serotonergic neurotoxins where placed in the para-position of an otherwise unsubstituted (on the ring) amphetamine. Possibly any highly electron-withdrawing functional group para to the alkylamino sidechain if the phenyl ring bears otherwise only hydrogen; I certainly wouldn't be taking any of them,
Which isn't to say you want either to happen. This holds true only for alkali metal and quite likely alkaline earth metal azides. Heavy metal azide salts like lead azide are sensitive, impact-sensitive, friction-sensitive etc. high explosives, very sensitive, known as primary explosives, lead azide having been used like mercury fulminate has, in the primers of gun cartridges that are located in the back of the round and which are impacted by the firing pin or hammer of the firearm, heavy metal azides, as well as IIRC transition metal azides are sensitive primary high explosives. As well as being toxic of course.
Hydrazoic acid itself is unstable. Not so unstable as not to exist, but its a sensitive explosive, and in all but dilute solutions it is highly explosive. I kinda think of it as 'hydrogen cyanide/hydrocyanic acid that explodes too'. Also more difficult to treat cases of poisoning than with cyanides. In the case of cyanide poisoning it is possible to treat it, first with an inhaled alkyl nitrite, to keep you alive long enough to administer the rest of the antidote, consisting of intravenous sodium nitrite (not nitrate, nitrIte) then thiosulfate, the nitrites convert haemoglobin to methhaemoglobin, disrupting the ability of cyanide anion to bind to haemoglobin's iron center (CN- has great siderophilic tendencies, a huge affinity for iron depending on the oxidation state of the iron), and then the cyanide is mopped up by thiosulfate, which is converted to thiocyanate and excreted. vitamin B12, as the hydroxocobalamin form is also used (B12 has several forms, including hydroxocobalamin and cyanocobalamin).
The thiocyanate, unlike cyanide, can be dialyzed, too, helping the body to get rid of it, although it is nowhere NEAR as toxic as cyanide.
Granted in cases of cyanide poisoning, treatment has to be extremely rapid, given how rapidly cyanide can poison someone fatally, but in the case of poisoning by azide, it can't be treated with a cyanokit,despite its also borking up the electron transport chain and inhibiting cellular respiration, azide poisoning is far more difficult to treat.
Talking of bioisosteres Pomzazed, can you perhaps point me towards any books on the subject, either that you know of download links for, or that I can grab with sci-hub or LibGen?
I'm very interested indeed on this subject, what can pose as a bioisostere for what, and especially with your mentioning that some groups only act as bioisoteres for other groups within certain specific contexts. I'd like to learn more about both, both bioisosteres in general, and what the limits are, where something one would think to be a bioisostere for a functional group, would in fact, in a specific context, not in fact, do so.
S.J.B.
Bluelight Crew
- Joined
- Jan 22, 2011
- Messages
- 6,886
Talking of bioisosteres Pomzazed, can you perhaps point me towards any books on the subject, either that you know of download links for, or that I can grab with sci-hub or LibGen?
I'm very interested indeed on this subject, what can pose as a bioisostere for what, and especially with your mentioning that some groups only act as bioisoteres for other groups within certain specific contexts. I'd like to learn more about both, both bioisosteres in general, and what the limits are, where something one would think to be a bioisostere for a functional group, would in fact, in a specific context, not in fact, do so.
You might find the following documents worth a look:
Comprehensive Medicinal Chemistry II, Volume 2, Chapter 16 (Bioisosterism) (2007)
Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design (J. Med. Chem. 2018 )
An interesting bioisostere which I saw mention of in a recent synthetic methodology paper is the replacement of a carbonyl with a gem-difluoroalkene moeity. It could be potentially used to modify cathinones:
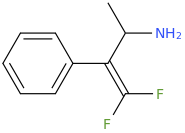
Last edited:
@Limpet
I dont really have a book, mostly using collective experiences. The link provided by SJP are great.
Basically, what is needed is
- mode of binding (what interaction is for the position)
- pocket volume
- special preferences inside the pocket (like electron density in specific posiiton)
Most of these info can be approximated from studied SAR (or better QSAR) of each class of compounds.
For example, cannabinoids, (ref THC) the has 3 different preferences of hydrophobic pockets
- the phenol part binds via pi stacking, so replacing it with things without p orbital dont work
- the terpene part binds via hydrophobic interactn, but “likes” some pi stacking, so replacing it with naphthalene works (like in JWH018); but replacing it with no p orbital hydrophobe bulk like adamantyl will also work
- the tail part binds via hydrophobe but dont like pi stack, etc
So that determines isostere that can be used at each posi.
@SJP
Nice readings up there! Appreciate it.
Also, that difluoroene part can replace carbonyl in many cases
I dont really have a book, mostly using collective experiences. The link provided by SJP are great.
Basically, what is needed is
- mode of binding (what interaction is for the position)
- pocket volume
- special preferences inside the pocket (like electron density in specific posiiton)
Most of these info can be approximated from studied SAR (or better QSAR) of each class of compounds.
For example, cannabinoids, (ref THC) the has 3 different preferences of hydrophobic pockets
- the phenol part binds via pi stacking, so replacing it with things without p orbital dont work
- the terpene part binds via hydrophobic interactn, but “likes” some pi stacking, so replacing it with naphthalene works (like in JWH018); but replacing it with no p orbital hydrophobe bulk like adamantyl will also work
- the tail part binds via hydrophobe but dont like pi stack, etc
So that determines isostere that can be used at each posi.
@SJP
Nice readings up there! Appreciate it.
Also, that difluoroene part can replace carbonyl in many cases
Bagseed
Bluelighter
- Joined
- Jul 22, 2010
- Messages
- 4,042
Damn, someone's salty it seems.You've ALREADY posted the whole gamut of ring-trihalogenated versions of various amphetamines. What is the point in posting the same things multiple times?
Oh, and for a whole load of this crap (admittedly you seem to actually be putting effort into coming up with things with a reason, or at least, intended reasoning, behind them now, which is good), there is no logic or wisdom behind it. There is a lot of just drawing pictures, giving them some ridiculous name and a load of utter bollocks about deities. That is pointless in the extreme, serving nobody, not us, and not you either. Thats just garbage, all that fucking horse-shit about deities.) And it certainly ain't current IUPAC, unless they have DRASTICALLY changed their system within the time this post first started.
And the para-haloamphetamines, dresden, with the exclusion of fluorine, aren't just bad for people, they are blatant neurotoxins. And they are used via microinjection into brain regions in animal studies, to cause permanent lesions of the serotonergic pathways targeted. Not just nasty, but outright poisons.
And whoever released PCA on the RC market needs to have a broken chair stuffed up their arse. Diagonally.
PCA at one point was IIRC released by big pharma as an antidepressant candidate, until it was found out the non-fluoro para-halogenated amphetamines (with no other substitution, E.g the likes of 2C-C. DOC, DOB, DOI etc.) were dangerous and long-term neurotoxins. They would probably also cause severe, lasting depression/crashes given the para-halo (excl. F) amphetamines cause a massive drop in serotonin production. Downright unpleasant little fuckers, and none of them should ever see the light of day outside of a lab (and IMO I find it pretty fucking disgusting they ever see the light of day *IN* one, at least in animal studies, its sick and twisted. Should stay as reference standards and possibly precursors) but otherwise, thats some sick, sick shit in my book. Yes, I realise why animal research is done, doesn't mean I have to like or agree with it)
Also, be aware of the existence of what are known as pseudohalogens, bioisosteres and often chemically resembling halogens, such as cyano, nitro groups, thiocyanato, pentafluorosulfanyl amongst others. Azide would be another, but you don't ever want an azido group on a drug of recreational intent, azido groups are unstable and make invariably for covalent, irreversibly binding ligands, splitting off N2 to form an amine. Can be useful in syntheses to produce amines (with care for azides are toxic, inhibiting the electron transport chain, like cyanide anion does, and those used in synthesis, usually sodium azide, NaN3, or other alkali metal azides, are somewhat explosive, and sensitive in the solid state, although they deflagrate rather than detonate (I.e burn fast, lots of hot expanding gases produced, they use sodium azide in car airbag charges, along with additives to mop up the sodium metal that is also liberated during a car crash when the charge goes off to inflate the airbag), going off as gunpowder does, rather than as a high explosive (I.e brisant, detonating not due to a fuel-oxidizer mix,the molecule of high-explosive flying apart releasing the large amounts of chemically stored energy, detonating, at a far higher speed than a deflagrating fuel-oxidizer 'low explosive' like gunpowder or flash powders.)
So things like para-nitroamphetamine, para-cyanoamphetamine would almost certainly be serotonergic neurotoxins where placed in the para-position of an otherwise unsubstituted (on the ring) amphetamine. Possibly any highly electron-withdrawing functional group para to the alkylamino sidechain if the phenyl ring bears otherwise only hydrogen; I certainly wouldn't be taking any of them,
Which isn't to say you want either to happen. This holds true only for alkali metal and quite likely alkaline earth metal azides. Heavy metal azide salts like lead azide are sensitive, impact-sensitive, friction-sensitive etc. high explosives, very sensitive, known as primary explosives, lead azide having been used like mercury fulminate has, in the primers of gun cartridges that are located in the back of the round and which are impacted by the firing pin or hammer of the firearm, heavy metal azides, as well as IIRC transition metal azides are sensitive primary high explosives. As well as being toxic of course.
Hydrazoic acid itself is unstable. Not so unstable as not to exist, but its a sensitive explosive, and in all but dilute solutions it is highly explosive. I kinda think of it as 'hydrogen cyanide/hydrocyanic acid that explodes too'. Also more difficult to treat cases of poisoning than with cyanides. In the case of cyanide poisoning it is possible to treat it, first with an inhaled alkyl nitrite, to keep you alive long enough to administer the rest of the antidote, consisting of intravenous sodium nitrite (not nitrate, nitrIte) then thiosulfate, the nitrites convert haemoglobin to methhaemoglobin, disrupting the ability of cyanide anion to bind to haemoglobin's iron center (CN- has great siderophilic tendencies, a huge affinity for iron depending on the oxidation state of the iron), and then the cyanide is mopped up by thiosulfate, which is converted to thiocyanate and excreted. vitamin B12, as the hydroxocobalamin form is also used (B12 has several forms, including hydroxocobalamin and cyanocobalamin).
The thiocyanate, unlike cyanide, can be dialyzed, too, helping the body to get rid of it, although it is nowhere NEAR as toxic as cyanide.
Granted in cases of cyanide poisoning, treatment has to be extremely rapid, given how rapidly cyanide can poison someone fatally, but in the case of poisoning by azide, it can't be treated with a cyanokit,despite its also borking up the electron transport chain and inhibiting cellular respiration, azide poisoning is far more difficult to treat.
Talking of bioisosteres Pomzazed, can you perhaps point me towards any books on the subject, either that you know of download links for, or that I can grab with sci-hub or LibGen?
I'm very interested indeed on this subject, what can pose as a bioisostere for what, and especially with your mentioning that some groups only act as bioisoteres for other groups within certain specific contexts. I'd like to learn more about both, both bioisosteres in general, and what the limits are, where something one would think to be a bioisostere for a functional group, would in fact, in a specific context, not in fact, do so.
Damn, someone's salty it seems.
Limpet_Chicken just have very high ionic strength,
Werd to mutha, I really don't care if these give you brain damage or not. If you don't want your brain, which has intrinsic plasticity, to evolve (or 'change') over time, then don't take psychotropic medications at all, including prescription antidepressants for example
Can't tell if serious or ironic.
Then again, I can't tell if Dresden named himself for the wizardly main character of the Dresden Files novels, or the German city known mostly for being thoroughly burnt-out after WW2
Re: Using a carbomethoxy group as a biostere for phenyl, it does work for remifentanil, being more than a 100 times the potency of morphine. Of course, this becomes less impressive when you consider that remifentanil is an analogue of carfentanil, which is approximately 10,000 times the potency of morphine.
Interestingly, it is also possible to replace the 4-carbomethoxy on carfentanil with a phenyl ring and get 4-phenyl-fentanyl, which is roughly 8 times the potency of fentanyl, i.e. more potent than remifentanil, but a far cry from the 100-fold increase in potency that the carbomethoxy groups confers in the 4-position. The 4-heteroaryl analogues are apparently more potent than the 4-phenyl, presumably because they are a better match for the electron distribution of the carbomethoxy.
https://en.wikipedia.org/wiki/4-Phenylfentanyl
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
The name came from the city.
I mean there's CH3CH2OH neurotoxicity and then there's HCN (g) NEUROTOXICITY, you know what I'm sayin? Nothing bad happened to the bluelighter who ate PCA.
This next one's IFFY if it doesn't work, but JIFFY if it does. Looks IFFY to me though.
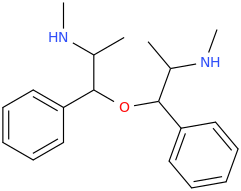
(J)IFFY
Hodor,
What do you think of this?
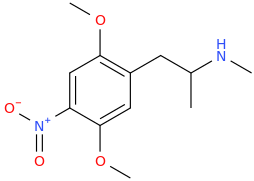
JASON
In case of emergency, call Jason!
* * *
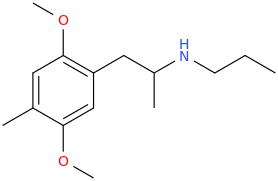
HODOR
Might take 100 mg. I really have ningun idea.
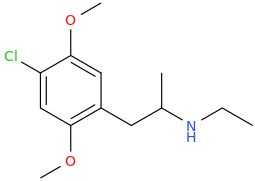
MISS MARIE "MS PACMAN" HANDBERRY
Might take 50mg. Una vez mas, yo no se.

TOOTY FRUITY (4-carbomethoxyphenol)
-vaginal juice extract
-topical male aphrodisiac
-used in many sunscreens
I mean there's CH3CH2OH neurotoxicity and then there's HCN (g) NEUROTOXICITY, you know what I'm sayin? Nothing bad happened to the bluelighter who ate PCA.
This next one's IFFY if it doesn't work, but JIFFY if it does. Looks IFFY to me though.

(J)IFFY
Hodor,
What do you think of this?

JASON
In case of emergency, call Jason!
* * *

HODOR
Might take 100 mg. I really have ningun idea.

MISS MARIE "MS PACMAN" HANDBERRY
Might take 50mg. Una vez mas, yo no se.

TOOTY FRUITY (4-carbomethoxyphenol)
-vaginal juice extract
-topical male aphrodisiac
-used in many sunscreens
Last edited by a moderator:
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
You've read the dresden files too? like it. Have you read Jim Butcher's other series, the codex alera? worth a shot if you liked the dresden files.
But it is pretty obvious the OP of this thread is just a burnt out heap of shit. Or at least, in dire need in being hit with a fuckton of white phosphorus and a couple of thermite shells rammed up some unlikely holes he probably doesn't even know the names of. Sure as shit isn't a fuckin' wizard.
The word 'wizard' is cognate with 'wise' and probably with 'wyrd', (as in the name of the rune), dresden is just gigantic example of a broken dildo that stinks of pentaborane and who needs to be soaked in a hot, saturated solution of white phosphorus in carbon disulfide. Wait, sod that, make it carbon diselenide, because like dresden, the pentaborane is poisonous muck that fucking stinks like something rotten crawled into a pot of yoghurt, crapped it's underwear and died and needs to fucking burn. And CSe2 just stinks.
The bipedal, mammallian equivalent to a tick,a disgusting, bloated tick, dug into the head of a mangy street dog's festering diseased dick and sucking all manner of noxious bodily excretions. If it weren't for the trouble I had to go to to make the stuff safely, I'd happily tip my bottle of iodine monochloride all over him, just to see if he autoignites.His posts are getting WORSE if that is actu ally possible, it seems!
Oh, and THANKS for the books on bioisostereism.
Also, wouldn't a gem-difluoroalkene be likely to act as some less than stable molecular add-oh, at the alkene end and get metabolized to something nasty like fluorophosgene, possibly or other unpleasant entities? I've never worked with fluorophosgene, and I don't want anything to do with phosgene itself; I can't imagine fluorophosgene being pleasant.And fluoroalkenesl like that just don't look at all pleasant, I'll be making sure to read the everloving mother of crap out of those publications Just the sort of thing I've been looking for and very much appreciated.
But it is pretty obvious the OP of this thread is just a burnt out heap of shit. Or at least, in dire need in being hit with a fuckton of white phosphorus and a couple of thermite shells rammed up some unlikely holes he probably doesn't even know the names of. Sure as shit isn't a fuckin' wizard.
The word 'wizard' is cognate with 'wise' and probably with 'wyrd', (as in the name of the rune), dresden is just gigantic example of a broken dildo that stinks of pentaborane and who needs to be soaked in a hot, saturated solution of white phosphorus in carbon disulfide. Wait, sod that, make it carbon diselenide, because like dresden, the pentaborane is poisonous muck that fucking stinks like something rotten crawled into a pot of yoghurt, crapped it's underwear and died and needs to fucking burn. And CSe2 just stinks.
The bipedal, mammallian equivalent to a tick,a disgusting, bloated tick, dug into the head of a mangy street dog's festering diseased dick and sucking all manner of noxious bodily excretions. If it weren't for the trouble I had to go to to make the stuff safely, I'd happily tip my bottle of iodine monochloride all over him, just to see if he autoignites.His posts are getting WORSE if that is actu ally possible, it seems!
Oh, and THANKS for the books on bioisostereism.
Also, wouldn't a gem-difluoroalkene be likely to act as some less than stable molecular add-oh, at the alkene end and get metabolized to something nasty like fluorophosgene, possibly or other unpleasant entities? I've never worked with fluorophosgene, and I don't want anything to do with phosgene itself; I can't imagine fluorophosgene being pleasant.And fluoroalkenesl like that just don't look at all pleasant, I'll be making sure to read the everloving mother of crap out of those publications Just the sort of thing I've been looking for and very much appreciated.
The name came from the city.
I mean there's CH3CH2OH neurotoxicity and then there's HCN (g) NEUROTOXICITY, you know what I'm sayin? Nothing bad happened to the bluelighter who ate PCA.
You can't really gauge neurotoxicity from self-reports, unless there are obvious physiological effects (ex.: MPTP-contaminated MPPP).
Yeah, a single dose of PCA is not going to burn out every last serotonergic neuron, but if something is deliberately used for its neurotoxicity in lab animals, I'd be *very* skeptical about repeatedly using it at recreational doses.
This next one's IFFY if it doesn't work, but JIFFY if it does. Looks IFFY to me though.

(J)IFFY
I call it POP.
Because it looks like someone tried to come up with an alternate synthesis route to methadone and ended up with a dimer that should be classed as a Persistent Organic Pollutant.