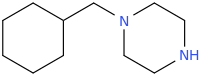
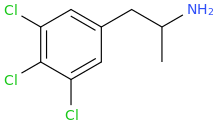
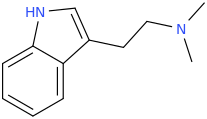
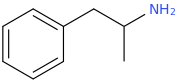
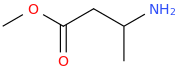
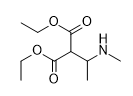
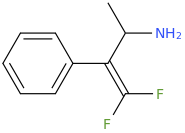
You've ALREADY posted the whole gamut of ring-trihalogenated versions of various amphetamines. What is the point in posting the same things multiple times?
Oh, and for a whole load of this crap (admittedly you seem to actually be putting effort into coming up with things with a reason, or at least, intended reasoning, behind them now, which is good), there is no logic or wisdom behind it. There is a lot of just drawing pictures, giving them some ridiculous name and a load of utter bollocks about deities. That is pointless in the extreme, serving nobody, not us, and not you either. Thats just garbage, all that fucking horse-shit about deities.) And it certainly ain't current IUPAC, unless they have DRASTICALLY changed their system within the time this post first started.
And the para-haloamphetamines, dresden, with the exclusion of fluorine, aren't just bad for people, they are blatant neurotoxins. And they are used via microinjection into brain regions in animal studies, to cause permanent lesions of the serotonergic pathways targeted. Not just nasty, but outright poisons.
And whoever released PCA on the RC market needs to have a broken chair stuffed up their arse. Diagonally.
PCA at one point was IIRC released by big pharma as an antidepressant candidate, until it was found out the non-fluoro para-halogenated amphetamines (with no other substitution, E.g the likes of 2C-C. DOC, DOB, DOI etc.) were dangerous and long-term neurotoxins. They would probably also cause severe, lasting depression/crashes given the para-halo (excl. F) amphetamines cause a massive drop in serotonin production. Downright unpleasant little fuckers, and none of them should ever see the light of day outside of a lab (and IMO I find it pretty fucking disgusting they ever see the light of day *IN* one, at least in animal studies, its sick and twisted. Should stay as reference standards and possibly precursors) but otherwise, thats some sick, sick shit in my book. Yes, I realise why animal research is done, doesn't mean I have to like or agree with it)
Also, be aware of the existence of what are known as pseudohalogens, bioisosteres and often chemically resembling halogens, such as cyano, nitro groups, thiocyanato, pentafluorosulfanyl amongst others. Azide would be another, but you don't ever want an azido group on a drug of recreational intent, azido groups are unstable and make invariably for covalent, irreversibly binding ligands, splitting off N2 to form an amine. Can be useful in syntheses to produce amines (with care for azides are toxic, inhibiting the electron transport chain, like cyanide anion does, and those used in synthesis, usually sodium azide, NaN3, or other alkali metal azides, are somewhat explosive, and sensitive in the solid state, although they deflagrate rather than detonate (I.e burn fast, lots of hot expanding gases produced, they use sodium azide in car airbag charges, along with additives to mop up the sodium metal that is also liberated during a car crash when the charge goes off to inflate the airbag), going off as gunpowder does, rather than as a high explosive (I.e brisant, detonating not due to a fuel-oxidizer mix,the molecule of high-explosive flying apart releasing the large amounts of chemically stored energy, detonating, at a far higher speed than a deflagrating fuel-oxidizer 'low explosive' like gunpowder or flash powders.)
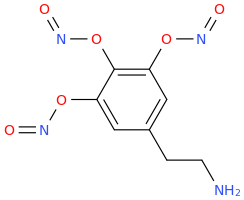
So things like para-nitroamphetamine, para-cyanoamphetamine would almost certainly be serotonergic neurotoxins where placed in the para-position of an otherwise unsubstituted (on the ring) amphetamine. Possibly any highly electron-withdrawing functional group para to the alkylamino sidechain if the phenyl ring bears otherwise only hydrogen; I certainly wouldn't be taking any of them,
Which isn't to say you want either to happen. This holds true only for alkali metal and quite likely alkaline earth metal azides. Heavy metal azide salts like lead azide are sensitive, impact-sensitive, friction-sensitive etc. high explosives, very sensitive, known as primary explosives, lead azide having been used like mercury fulminate has, in the primers of gun cartridges that are located in the back of the round and which are impacted by the firing pin or hammer of the firearm, heavy metal azides, as well as IIRC transition metal azides are sensitive primary high explosives. As well as being toxic of course.
Hydrazoic acid itself is unstable. Not so unstable as not to exist, but its a sensitive explosive, and in all but dilute solutions it is highly explosive. I kinda think of it as 'hydrogen cyanide/hydrocyanic acid that explodes too'. Also more difficult to treat cases of poisoning than with cyanides. In the case of cyanide poisoning it is possible to treat it, first with an inhaled alkyl nitrite, to keep you alive long enough to administer the rest of the antidote, consisting of intravenous sodium nitrite (not nitrate, nitrIte) then thiosulfate, the nitrites convert haemoglobin to methhaemoglobin, disrupting the ability of cyanide anion to bind to haemoglobin's iron center (CN- has great siderophilic tendencies, a huge affinity for iron depending on the oxidation state of the iron), and then the cyanide is mopped up by thiosulfate, which is converted to thiocyanate and excreted. vitamin B12, as the hydroxocobalamin form is also used (B12 has several forms, including hydroxocobalamin and cyanocobalamin).
The thiocyanate, unlike cyanide, can be dialyzed, too, helping the body to get rid of it, although it is nowhere NEAR as toxic as cyanide.
Granted in cases of cyanide poisoning, treatment has to be extremely rapid, given how rapidly cyanide can poison someone fatally, but in the case of poisoning by azide, it can't be treated with a cyanokit,despite its also borking up the electron transport chain and inhibiting cellular respiration, azide poisoning is far more difficult to treat.
Talking of bioisosteres Pomzazed, can you perhaps point me towards any books on the subject, either that you know of download links for, or that I can grab with sci-hub or LibGen?
I'm very interested indeed on this subject, what can pose as a bioisostere for what, and especially with your mentioning that some groups only act as bioisoteres for other groups within certain specific contexts. I'd like to learn more about both, both bioisosteres in general, and what the limits are, where something one would think to be a bioisostere for a functional group, would in fact, in a specific context, not in fact, do so.