Bagseed
Bluelighter
For my THC-infused mind it was tldr unfortunately. 
N&PD Moderators: Skorpio | someguyontheinternet
Werd to mutha, I really don't care if these give you brain damage or not. If you don't want your brain, which has intrinsic plasticity, to evolve (or 'change') over time, then don't take psychotropic medications at all, including prescription antidepressants for example
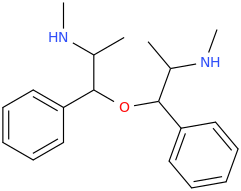
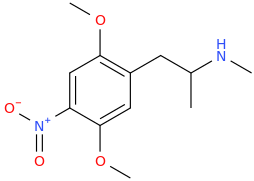
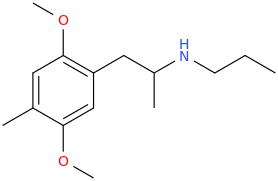
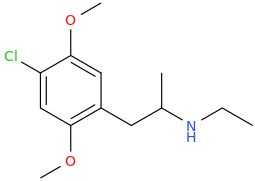

The name came from the city.
I mean there's CH3CH2OH neurotoxicity and then there's HCN (g) NEUROTOXICITY, you know what I'm sayin? Nothing bad happened to the bluelighter who ate PCA.
This next one's IFFY if it doesn't work, but JIFFY if it does. Looks IFFY to me though.

(J)IFFY
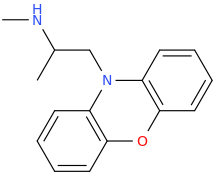
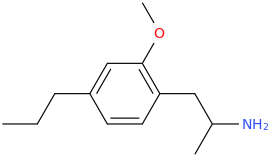
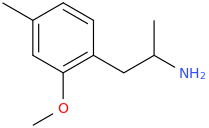
They latter two are not CB agonists, certainly not pure ones anyway. Are you familiar with promethazine? It's a sedative. I realize that likely implies H1 antagonism.
By Cronos, I apparently know a lot more than anyone gives me credit for!
"The Greatest Intelligence Seems Stupid."--Lao Tzu.