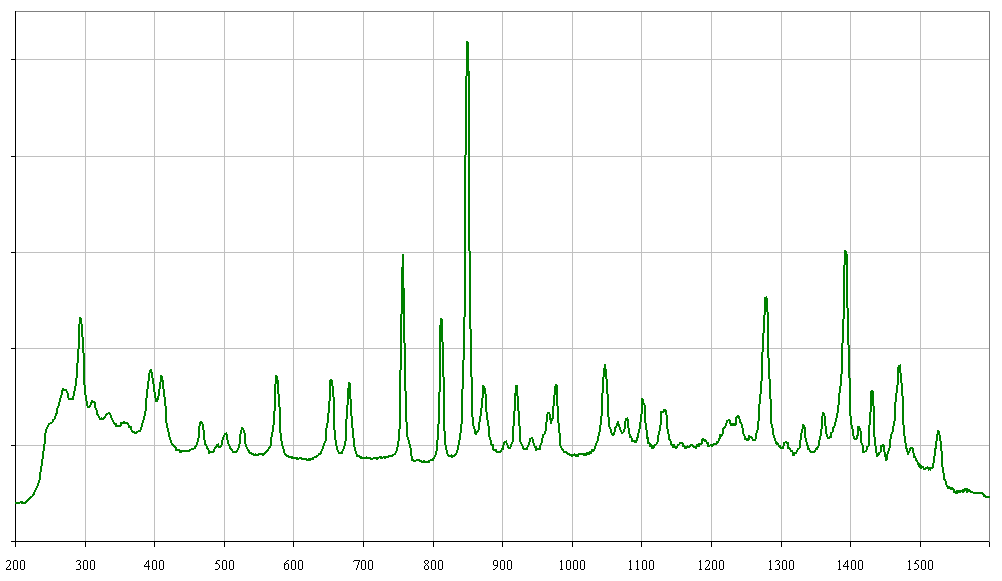
It looks like I found it, too! This week I bought 1g of MDMA HCl clear crystals locally made via the Leuckart reaction (at least that's what the seller says) . Yesterday, I tested 90mg of it on MDMA-naïve 29y.o., 62kg female with no history of stimulant or antidepressant use. I observed:
20min onset, 5h duration, 86% mydriasis, visible trismus, 37.2ºC, 150/100 b.p., 110bpm., energetic, open and plush behavior, responsive to music (danced 1h) later slithering and seeking touch. Blood glucose level elevated 220% @ 2h despite fasting 3h before and during and only salted water consumed. No info on comedown yet.
Stokes spectrogram is below:
A solution of this product does not exhibit any optical rotation in my polarimeter.