OK, I'm begging again:
https://doi.org/10.1021/acs.joc.8b01673
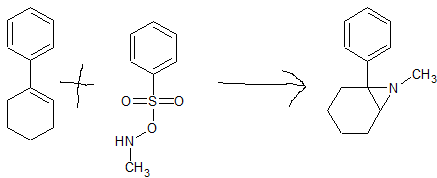
Direct N-H/N-Me Aziridination of Unactivated Olefins Using O-(Sulfonyl)hydroxylamines as Aminating Agents
Just so people realise, I AM aware that the quality of many papers of Indian origin are very dubious. Anyone else remember the fake '1-pot synthesis of fentanyl' that was someone's pHd work (and got retracted since it was made up) BUT:
1)This is published in the JOC, a high impact journal.
2)The researchers have worked on several papers on aziridines so it seems they have developed a knowledge bade.
3)They cite and are cited appropriately and not just other people within their own department (a la Jan Hendrik Schön AKA Nobel Scandal)
4)A totally different team developed a related approach (N-phenoxy) while they use an animoxy
So why the hassle?
Image aaa hosted in ImgBB

ibb.co
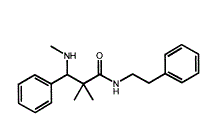
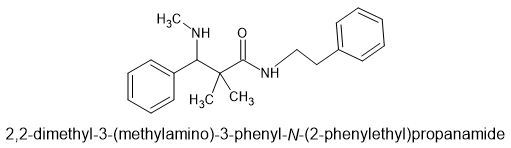
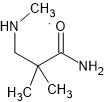
OK, so you likely wonder what the heck is the point of THAT? Well, it's because that 3-membered ring is under a lot of strain and so it will ring-open to an alkanolamine or an aminoester or an aminoketone or an aminoalkylsulfonate or... well MANY things. What is even better is that the ring-opening will be almost 100% trans. I mean 99.8% ee or better (i.e. >99.9% trans)
Now, if one were to apply it to say (a ring-substituted) propenylbenzene, you could produce optically pure (ring-substituted) pseudoephedrine. I just use those as examples. I do not see any advantage in that since IF you wanted those, it's only of great benefit when the precursors are costly.
BTW the catalyst for THIS reaction is Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)]. The catalyst costs £400/gram so you can see it's only when the precursor is EXPENSIVE. It is actually a very interesting example of how a catalyst works. When you get that far down the periodic table, you can get some wacky oxidation states. Rh can be +1, +2, +3, +4, +5, +6 and the catalyst has a LOT of steric bulk and so it works by promoting an electron (HOMO/LUMO theory).
It get's VERY complex. I am hoping that their will be alternatives BUT I need the article to test.