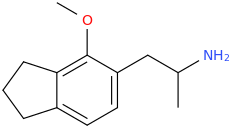
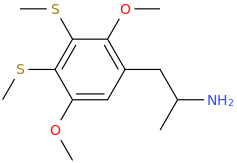
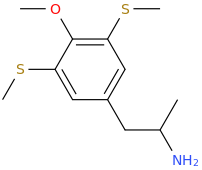
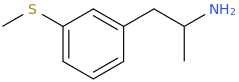
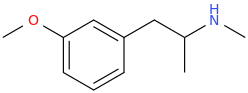
It's been made. Pubchem points to a list of controlled compounds containing it. The1-(4,7-Dimethoxy-2,3-dihydro-1
H-inden-5-yl)propan-2-amine analogue (obviously with the extra 5-methoxy) is in the updated version of Pihkal as G-3.
From Shulgin's QSAR work, you don't get much 5HT2a affinity with just the 2-methoxy. The indaneamphetamine's, lacking the

also lack SERT affinity so you end up with a stimulant.
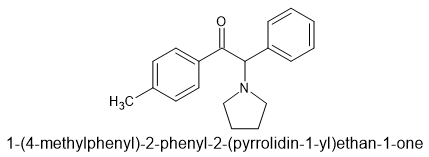
We worked on some that would evade MoDA and while the indane worked, the p-Me was better. Better because it's duration was shorter. Specifically we went with:
Image 1 hosted in ImgBB

ibb.co
As you can see, we found that an N-pentane chain could be exchanged for an ethyl and then a benzene ring. So in fact it's a bioisostere of pyrovalerone. It may look a lot different, but really all that has changed is that part of the alkyl chain has been swapped with an aromatic. Some other aromatics also work.
Well, we tried placing an indane at the 3,4 of the left-hand benzene but it turned out to produce something with a DAT/NET balance and pharmokinetics that meant it wasn't as good. The p-Me is a perfect sacrificial moiety. The body can so easily oxidise it so it's duration is only about 2 hours.