Found this, supposedly from one of the next big shulgin books,
From the working draft of an upcoming book by Shulgin, "Psychedelic Index", Transform Press, 2004
Names:
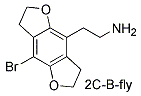
2C-B-FLY
1-(8-Bromo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran-4-yl)-2-aminoethane
Registry Numbers:
CARN
HCL salt 178557-21-6
Natural sources:
Not known in nature
Synthesis:
From hydroquinone (with 1-chloro-2-bromoethane) to 1,4-bis(2-chloroethoxy)benzene (with Br2) to 1,2-bis(2-chloroethoxy)-2,5-dibromobenzene (with BuLi) to 2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran (with dichloromethyl methyl ether) to 4-formyl-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran (with CH3NO2) to 4-(2-nitro-1-ethylene)-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran (with LAH) to 1-(2,3,6,7-Tetrahydrobenzo[1,2-b:4,5-b']difuran-4-yl)-2-aminoethane (with Br2) to 2C-B-FLY (Monte, et al., 1996).
History:
The "FLY" name was inspired by the two dihydrofuran rings that extend oppositely from the sides of the benzene ring. When they are aromatized (furan rings) they are planar with the benzene ring and the code name is "DRAGONFLY" and they are listed as DFLY derivatives.
Physical properties:
C12 H14 Br N O2
HCl salt m.p. >310 (EtOH, EtOAc) (Monte, et al. 1996).
Pharmacology:
The five "FLY" compounds (2C-FLY, 2C-B-FLY, FLY, B-FLY and DOM-FLY) were assayed in a drug discrimination paradigm with LSD-trained rats, and in interactions with various serotonin receptors. (Monte, et al., 1996).
2C-B-FLY is active in man at an oral dose of 10 milligrams (Shulgin, 2003a)
Legal Status:
2C-B-FLY is not listed in any law.