MedicinalUser247
Music Ambassador
- Joined
- Aug 2, 2023
- Messages
- 4,746
That looks cool.
N&PD Moderators: Skorpio | someguyontheinternet
Well it actully looks more like dopamine than that.
Is ephedrine stronger than meth?The thing is when you have two hydroxyl's like dopamine you can't make an amphetamine out of it because it's neurotoxic. That's why you need the Ephedrine structure as a substitute.
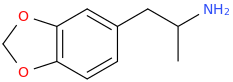
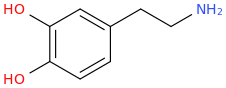
Of course meth is stonger, but adding two hydroxyl groups to meth makes it neurotoxic when you place them in the same position as dopamine.Is ephedrine stronger than meth?