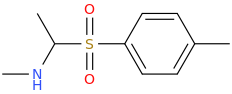
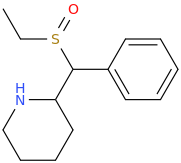
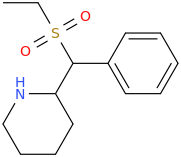
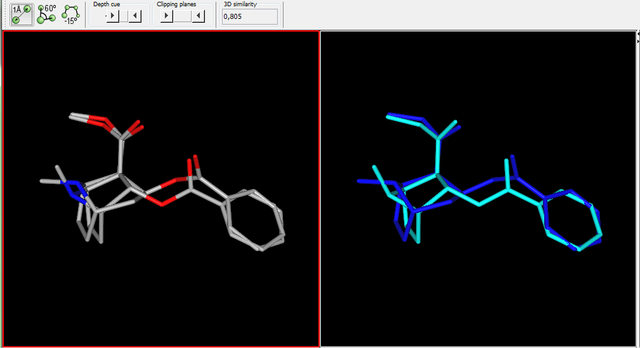
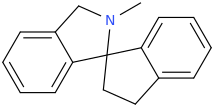
inspired by the many thio on this page... thio 4-FPM ..
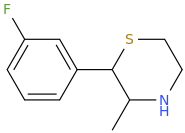
Now since sulfide are easily oxidized (even by simple exposure to ambient oxygen in air.. thio-4FPM-1-oxide:
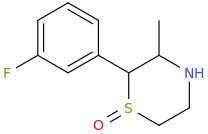
Now this group (sulfoxide) looks like one found in modafinil type stimulants used by military..so could be good nootropic functional stim cross between FPM+Modafinil.. but who knows?
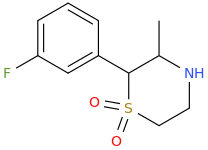
....for completion don't forget the S,S-dioxide:
nb: these are new untested compounds afaik (if you happen be in situation to be able to synthesize'em (pretty easy actually!) .. give me credit and send me my 3% patent royalties ..

Now since sulfide are easily oxidized (even by simple exposure to ambient oxygen in air.. thio-4FPM-1-oxide:

Now this group (sulfoxide) looks like one found in modafinil type stimulants used by military..so could be good nootropic functional stim cross between FPM+Modafinil.. but who knows?
....for completion don't forget the S,S-dioxide:

nb: these are new untested compounds afaik (if you happen be in situation to be able to synthesize'em (pretty easy actually!) .. give me credit and send me my 3% patent royalties ..
Last edited: