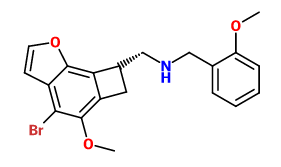
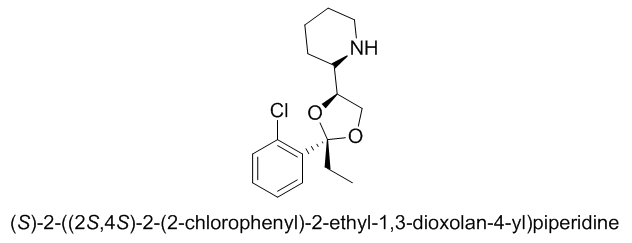
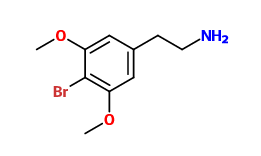
What's the deal with Bromine, from a biochemical standpoint? Why is it so prevalent in psychoactive chemicals?
Bromine is both electronegative (i.e. it draws in electrons, which may be important for interacting with receptors), and relatively large (which can greatly affect a drug's potency). It is also relatively easy to both attach to and selectively detach from a molecule, meaning you can easily synthesize a brominated compound *and* use it as a precursor for e.g. a chlorinated compound.
However, I wouldn't say that it is all that "common" in psychoactives. Sure, for the reasons mentioned above it is basically "perfect" for the 4-position in a psychedelic phenylethylamine (so you've got DOB, as well as its analogues 2C-B, 2C-B-FLY, 25B-NBOMe, and so on), but as far as other psychoactives are concerned, bromine is probably rarer than chlorine (note: fluorine, chlorine, bromine and iodine form a group of very reactive elements called "halogens" that play an important role in Organic Chemistry).
There's been relatively little interest in brominated tryptamines, for example.
With many "legal highs" or "research chemicals", people will even deliberately use fluorine instead of other halogen substituents like bromine because it is such a *small* molecule - para-Fluoro versions of fentanyl analogues, for example, probably aren't going to differ from their parent molecules all that much as far as potency and toxicity are concerned.
Last edited: