-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Volsam
Bluelighter
- Joined
- Nov 19, 2016
- Messages
- 909
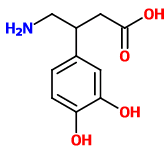
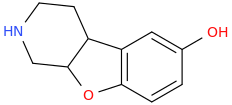
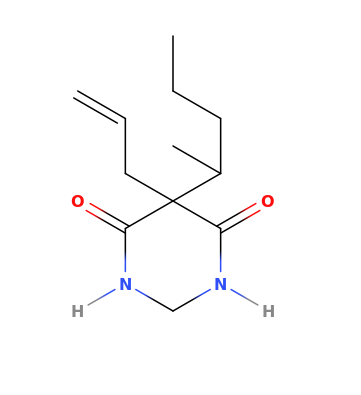
These seem obvious but I can't find any information about them. They're probably active, but maybe too difficult to synthesize.
^^^ They look yummy! :D
Look very similar to 2C-G-3 that has very nice comments and active at 16-25mg. Considering these two having a sulfur on 4th position, it might become up to 4 times as potent, so probably will be active at 5-10mg, am I right?..8)
And what about benzofuran (and dihydrobenzofuran) analogs of those (oxygen instead of a sulfur), were they ever made?..
cj187
Bluelighter
- Joined
- Dec 1, 2015
- Messages
- 635
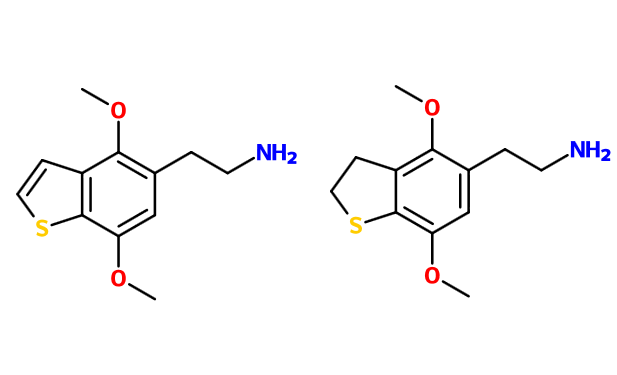
Here are two obscure chems briefly mentioned in Shulgin's lab notes:
"Active 25-30mg. 5 hrs. Good eyes-closed visuals."
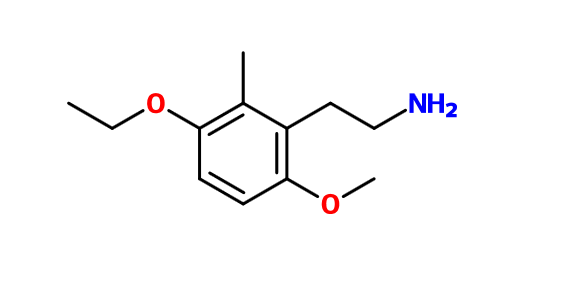
"doesn't have much re alteration of consciousness, but marvelous visuals - in light, squinting needs dark glasses, but no dilation. Wine cellar -> 100ft vision [with] detail."
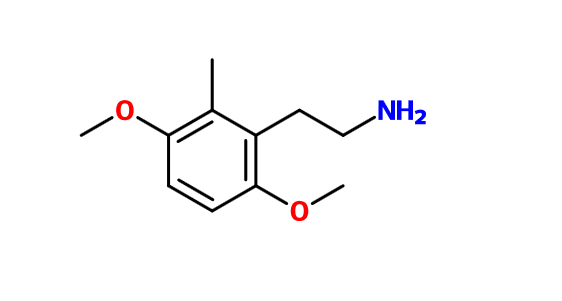
Clearly this one should be active too:
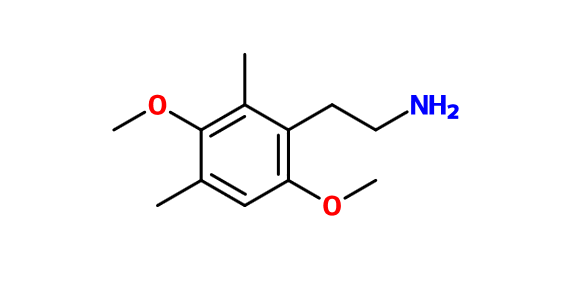
I would think that this would work too:
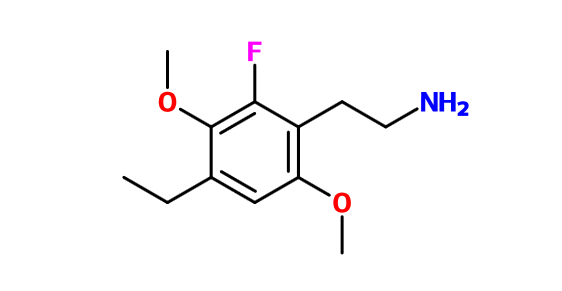
What if you put a halogen at the 6 position of instead of an alkyl group? 2-FA and 2-FMA are considered worthwhile drugs by many people. Perhaps you could do the same thing with the psychedelic phenethylamines.

"Active 25-30mg. 5 hrs. Good eyes-closed visuals."

"doesn't have much re alteration of consciousness, but marvelous visuals - in light, squinting needs dark glasses, but no dilation. Wine cellar -> 100ft vision [with] detail."
Clearly this one should be active too:

I would think that this would work too:

What if you put a halogen at the 6 position of instead of an alkyl group? 2-FA and 2-FMA are considered worthwhile drugs by many people. Perhaps you could do the same thing with the psychedelic phenethylamines.

Soulfake
Bluelighter
- Joined
- Aug 12, 2010
- Messages
- 160
I wonder why you shouldn´t be able to use a weak fentanyl-derivate which has a similar potency to Morphine, Oxycodone etc. as a recreational drug, why are people always into the strong fenta analogues? I imagine a weak one with a long half life to be really good.


Soulfake
Bluelighter
- Joined
- Aug 12, 2010
- Messages
- 160
Some carisoprodol-analogues. I wonder why none of those muscle relaxers have made it to the rc-marked, same with Gabapentin or Pregabalin-Analogues (which seem to be relatively easy to make, like esters, pro-drugs or small molecular changes, just look how simple the "rules" are with Gaba/Baclofen/Phenibut/Tolibut/Gabapentin/Pregabalin/GABOB etc.). I'm very sure many people would buy it even if not too many use them recreationally but as more people know about it more will test it out and it'll get a similar place to benzos in the relaxing-rc category. Of cause they are more dangerous chemicals than benzos but well, the train seems to have left as they sell stuf like NBOME, fentanyl-analogues, Bromadol etc. even in pure form, so a few muscle relaxers won't hurt the whole thing.


Last edited:
I wonder why you shouldn´t be able to use a weak fentanyl-derivate which has a similar potency to Morphine, Oxycodone etc. as a recreational drug, why are people always into the strong fenta analogues? I imagine a weak one with a long half life to be really good.
What's the point of developing one with morphine or oxy potency.. It can never be cheaper than the last 2, right? But just in case: IIRC the metabolite of fenta (without the phenethyl chain is as potent as morp or it is the metabolite of carfenta (have to check my notes). Nice structures (I like the last one especially if you make it bis-2-thiophenyl, furans are hard on the liver)
aspiringdrugdesign
Bluelighter
- Joined
- Jul 5, 2017
- Messages
- 67
Midnight Sun
Bluelighter
- Joined
- Oct 31, 2014
- Messages
- 71
Some carisoprodol-analogues. I wonder why none of those muscle relaxers have made it to the rc-marked, same with Gabapentin or Pregabalin-Analogues (which seem to be relatively easy to make, like esters, pro-drugs or small molecular changes, just look how simple the "rules" are with Gaba/Baclofen/Phenibut/Tolibut/Gabapentin/Pregabalin/GABOB etc.). I'm very sure many people would buy it even if not too many use them recreationally but as more people know about it more will test it out and it'll get a similar place to benzos in the relaxing-rc category. Of cause they are more dangerous chemicals than benzos but well, the train seems to have left as they sell stuf like NBOME, fentanyl-analogues, Bromadol etc. even in pure form, so a few muscle relaxers won't hurt the whole thing.

I love carisoprodol and would like to see more analogues, but carisoprodol is not very potent which is probably the greatest blockade to seeing analogues come to market -- second to that would be the fact that carisoprodol itself is hit or miss with people. Remember nifoxipam?
Barbiturates on the other hand... those suffer from impotency issues too, but not as bad as carisoprodol (I have to eat >2g to get anywhere with cariso) and they're more universally well-liked, albeit more lethal. Thing is at least in the US there's a catch-all on in the analogue act which schedules any derivative of barbituric acid. Now... desoxy analogues like desosecobarbital above are exempt since barbituric acid isn't used or made in the synthesis
 -- they act as prodrugs to the real deal, though
-- they act as prodrugs to the real deal, though What's the point of developing one with morphine or oxy potency.. It can never be cheaper than the last 2, right?
so people stop dying?
Make no mistake: fentanyl does NOT have a manufacturing cost anywhere NEAR the retail or bulk price. Because the strong potency is common knowledge the chinks charge according to effective dosage which you can't blame them for doing. OTOH if China wants to keep pissing strong opioids into the world supply they should start pumping out dipipanone or nitazene analogues instead
^ guess I didn't make my post clearer: I mean why not just take morphine or oxydocone if the idea is to supply opioids (fentanyl analogues) with morphine or oxy potency! Sure the manufacturing costs of fentanyl are near zero compared to the retail price. But it can never be cheaper than opiates derived from poppys seeds (you just plant the shit and harvest it like potatoes or broccoli!) IMHO, the whole issue is due to PROHIBITION which is really where most of the retail or bulk price for BOTH synthetic and natural opiates is.
doxylamine
Greenlighter
- Joined
- Aug 8, 2010
- Messages
- 47
Haha, "Dopa-but"
aspiringdrugdesign
Bluelighter
- Joined
- Jul 5, 2017
- Messages
- 67
(4R,4aR,7aR,12bS)-9-methoxy-3,4a-dimethyl-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one (4-methylhydrocodone)
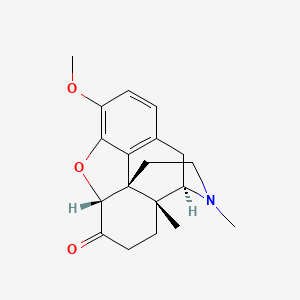
It is very similar to hydrocodone and oxycodone, except the hydrogen at 4a in hydrocodone and hydroxyl at 4a in oxycodone has been replaced by a methyl group. Would this compound be active, and where would the potency lie? My guess is less than hydro/oxycodone.
It is very similar to hydrocodone and oxycodone, except the hydrogen at 4a in hydrocodone and hydroxyl at 4a in oxycodone has been replaced by a methyl group. Would this compound be active, and where would the potency lie? My guess is less than hydro/oxycodone.
doxylamine
Greenlighter
- Joined
- Aug 8, 2010
- Messages
- 47









First contribution to this thread. Long time lurker and has given it me many laughs so time to return the favour! Had to include some methylenedioxy versions as part of the running joke here, naturally
EDIT: Gah, sorry the images are so huge. Will resize next time.
Last edited:
aspiringdrugdesign
Bluelighter
- Joined
- Jul 5, 2017
- Messages
- 67
That 2c-b-fly/cyclopropyl GABA mashup is pretty interesting
Soulfake
Bluelighter
- Joined
- Aug 12, 2010
- Messages
- 160
^ guess I didn't make my post clearer: I mean why not just take morphine or oxydocone if the idea is to supply opioids (fentanyl analogues) with morphine or oxy potency! Sure the manufacturing costs of fentanyl are near zero compared to the retail price. But it can never be cheaper than opiates derived from poppys seeds (you just plant the shit and harvest it like potatoes or broccoli!) IMHO, the whole issue is due to PROHIBITION which is really where most of the retail or bulk price for BOTH synthetic and natural opiates is.
I would love to just take same oxy or morphine but it is nearly impossible to obtain for me. The only time I got the chance to try morphine was when some friends brought those substitol capsules from Vienna that are used for opioid addiction there. 10 years ago I got a few oxy tabs but since then I never found it again. So for the RC-market some light opioids would be nice, desmethyl-Tramadol and AH7291 where quite popular and why not use some light fentanyl analogues (or similar light analogues of other kinds of usually strong opioids), they are cheap and easy to make and there are many different possible variations. Of cause some synthetic "natural looking" opioids would be the best (similar to the cannabinoids) but vendors just are too greedy to sell something that pricey and bulky if they can sell comparatively small amounts of stronger opioids that are also cheap to make.
Some analogues of the n-methylcarfentanyl which has about the potency of morphine:

Last edited:

These seem obvious but I can't find any information about them. They're probably active, but maybe too difficult to synthesize.
The problem is the synthesis. There all manner of likely compounds (and those look very good) but if you like to draw, extend to synthetic references. S can be a pain since it exhibits +2, +4 & +6 oxidation states. I have also struggled with the synthetic route. As a rule-of-thumb only 3 'pots' is the maximum. I spent a good 5 years reducing the synthesis of the reversed-ester of tilidine from 5 steps to 2. In fact, it was a 2016 paper that dealt with the issue. Consider U-47700. With the right precursors (acid chloride and 1,2,2-trimethyl aminocyclopropan-2-diamine). The former is a 'fine chemical' and the latter is commercially available.
I think that is the important thing. We can conceive of all manner of compound with very likely good activity but the mechanical losses when making a sample make some things expensive and the sheer precursor costs make bulk unattractive. For what it's worth, the aromatic is VERY likely to have activity but adding those methoxy groups makes it impossible.
Use Chemoffice to see if precursors are available.
CC
PS the thiophene analogs of mephedrone and related p-Me cathinones has been studied. Reaxys gives a lot of refs but once again, good luck finding the precursors and dealing with the stench during manufacture. I got the 2-Me benzaldehyde that was a pitch black liquid that smelt of dead people. That it went forward as a PS amazed me. The furan would be interesting. Reaxys also threw up the O analog of clomethiazole. The furan is more potent, longer lasting, is pH neutral for parenteral administration but as long as thiamine (hydrolysis of B1) is OTC, anyone with thionyl chloride can make Heminevrin. The hydrochloride produces big fluffy crystals but supplying something that managed to kill Keith Moon is obviously a set for disaster.
- Status
- Not open for further replies.