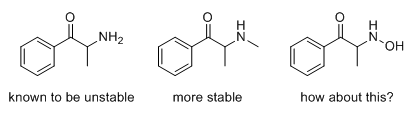
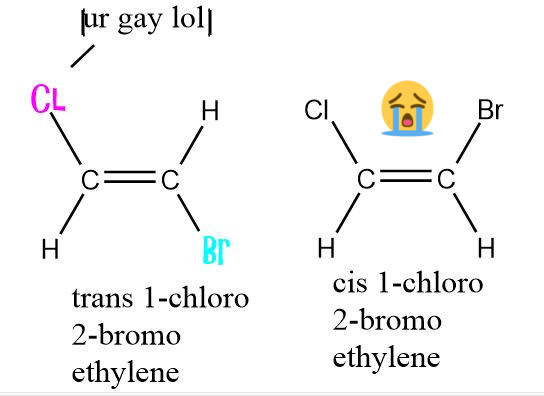
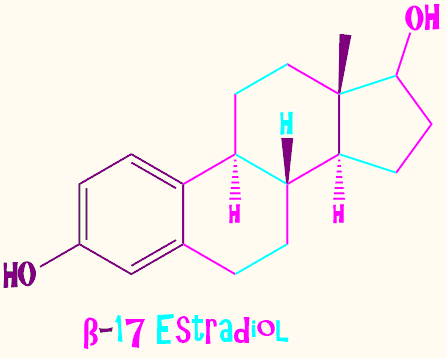
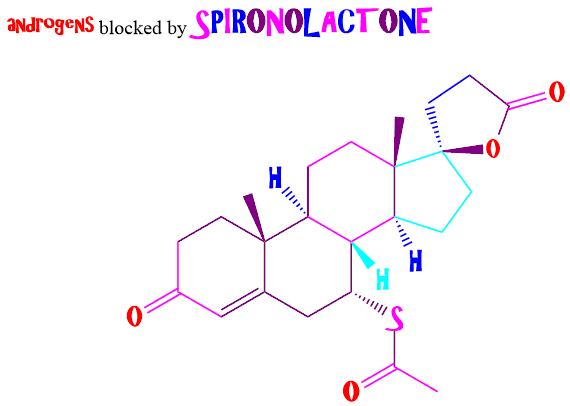
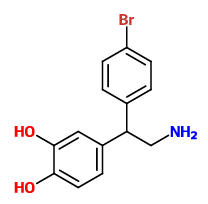
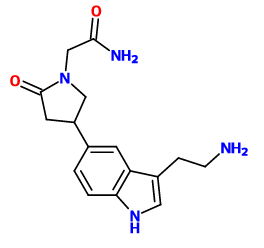
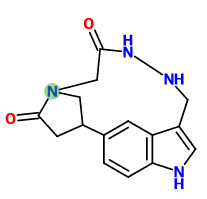
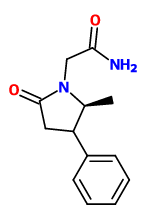
adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,852
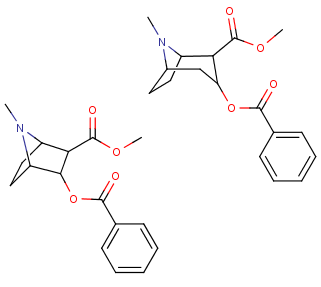
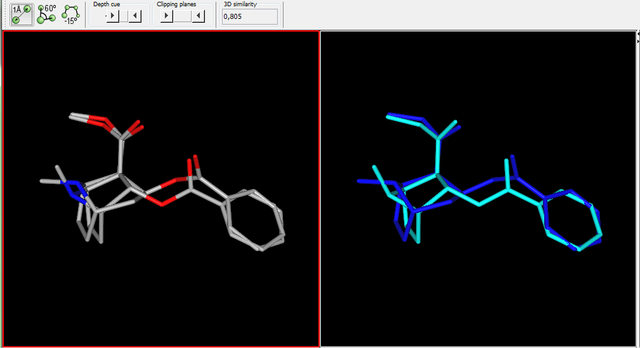
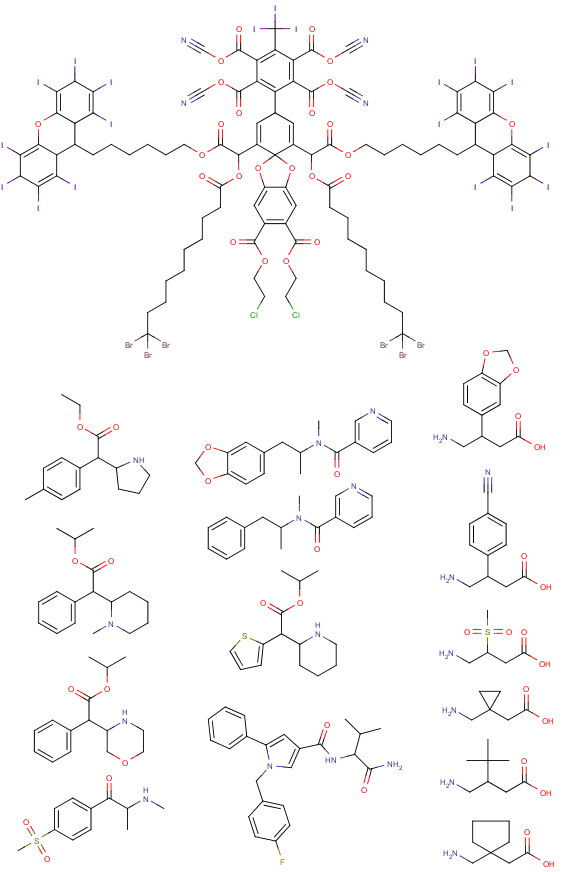
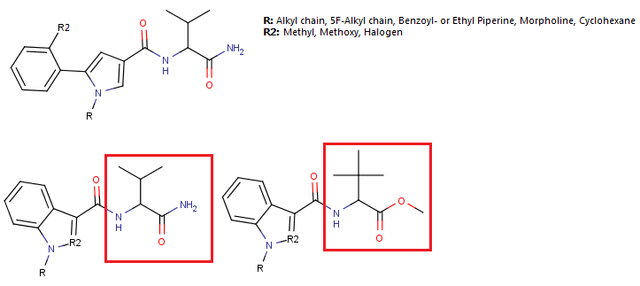
Reaxys does give you fairly neat tools to input information aside from searching with words and phrases, i.e. you can query with structures and reaction schemes which is very nice and if you're looking for a very specific reaction, searching with Reaxys is usually the fastest. But the structure editing tool is far from perfect IMO, the best structure editor is in Java and thus basically useless on modern browsers, all the rest is rather uncomfortable to use especially when you want to mark specific R groups and obtain information how broadly some reaction has been researched, but it can be done even if sometimes it requires a few queries instead of one. You definitely have no way of searching through articles like this with Scholar Google, you have to input specific words to get a list of publications and then look through those publications to see if they contain the reaction or compound you're looking for (which is rather pointless if you're given such a job to do, you should be given a more appropriate tool IMO). But still, Scholar Google is what I use at home to find articles on specific topics, IME it works pretty well and you can still find a lot of more rare articles in organic chemistry this way as well. If you're looking for some general information on some reaction as a general method, then Scholar Google is definitely enough, you find an article which links to a review and then it's easy to find more information available through references and knowing names of researchers through the search engine again. You never know if it's exhaustive, with Reaxys you can't be sure either, but it's what most people in academia use (I guess). Anyway, if you carry out research and have to compile data, then that's when Reaxys is indeed indispensable when you have to check if your compounds have already been synthesized and if yes, compare analytical data from the literature with yours, good luck doing that with Scholar Google. :D No matter how clumsy the structure editor in Reaxys may feel, it's million times faster to find specific data for a given organic compound with Reaxys than with any search engine which accepts only words and phrases. I don't really use PubMed too often nowadays, so I can't say much about it.
All in all, I would definitely not invest into Reaxys either unless I ran a private company and did research that required certain functionalities Reaxys does offer.
All in all, I would definitely not invest into Reaxys either unless I ran a private company and did research that required certain functionalities Reaxys does offer.