izo
Bluelighter
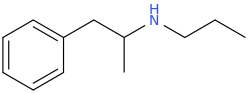
That's important in nations where Markush structures are used to decide if a compound is legal.
dont know the meaning of markush structure. but in germany 5/6-Apb is restricted under the NPSG as a ring substituted 2-PEA and therefor is only legal for scientific use.




![Spiro[3,5]nonane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2FSpiro%5B3%2C5%5Dnonane.png&hash=dafdc95c79727336f53c8b725de05c3c)
![spiro-[5,5]-undecane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2Fspiro-%5B5%2C5%5D-undecane.png&hash=6d8a6dcd569d208d37358549fbcfab80)