StoneHappyMonday
Bluelighter
- Joined
- May 10, 2001
- Messages
- 18,084
"Dove territory of the early nineties"



NiceI love you all!!! Orange Tealas are the best of all time. I'm still buzzing. I used to need 2 pills 6 weeks ago after Ibiza, so I started taking Piracetam and St. John's wort. It's reset my tolerance, a full one was almost too much and a 3 hour high. Tonight felt like the first time on mdma all over again.
Piracetam works!!!
 good to hear you're having fun, enjoy man! Lets see if I can find some of those teslas
good to hear you're having fun, enjoy man! Lets see if I can find some of those teslasI'm pretty sure thousands of people have come across brown MDMA, in the last day or two, never mind recently. There's no way that we can give any indication on the quality of your batch, as it's just crystals. At least with pills there's a chance of offering some insight as to what they're like...Have any of you guys come across brown mdma recently?? 'Dutch' by all accounts....
If so any good?
Thanks
The MDMA in the Rainbow Drops? I posted my opinion a page or two back. I think it's new stuff, and it's inferior. It could be me, but friends agreed. Still nice, but not as nice as they should be. I shall be stocking up on lions, as I enjoyed the time I tried them, but was also doing a lot of speed, which definitely affected the experience. I never expected there to be much cross-tolerance between amphetamine and MDMA, but there definitely is.Yes and they are Dutch. Can't really tell much from the pic mate but I'll take your word for it. From what a friend said the Dutch Lions are the best pills he had recently and he got them stupidly cheap, same friends are aware the UFO's etc were better than most of the Dutch pills. Rainbow drops apparently not the strongest, people saying they're only worth a fiver as you need to double drop...I'd be more interested to hear what the MDMA is like...
more of them Toyota MDMA/MDA combo http://www.saferparty.ch/tl_files/i...en_PDF_2015/MDMA+MDA_September_2015_Basel.pdf
Sidna: I suppose this shows my lack of chemistry knowledge, but would the addition of MDA, of the opposite isomer somehow balance things out, as it's a similar drug?
Valency normally refers to the charge on an ion i.e. how many electrons the neutral atom is missing or has extra. In the HCl form, the amine part of MDMA gains a + charged hydrogen from the H-Cl to give the amine a + charge, which forms an ionic bond with the negative Cl- ion.
I suggest reading some basic organic chemistry regarding isomers. Pick a book/online page etc that details isomers with good pictures. You don't have to learn it all, but a non in-depth reading will allow you to understand the terminology.
Several different types of isomers exist but not all are relevant to MDMA.
Molecules termed enantiomers, are stereoisomeric forms (isomers) related to each other as are your right and left hands. They are non superimposable images because of the shape resulting from 4 different groups attached to a chiral carbon centre.
Pic from wiki page on chirality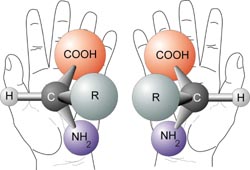
An example of a chirality is the second carbon on the alkyl branch of MDMA (yellow in below pic) that is bonded to:
1) a Nitrogen with 1 Hydrogen & a CH3 ( -NH-CH3 )
2) a Carbon with 3 hydrogens ( -CH3 )
3) a Carbon with 2 Hydrogens ( -CH2- )
4) a Hydrogen (-H )
Spatially, these 4 groups may be positioned differently. Steric repulsion allows by rotation, certain positions to be favoured, so placement of the chiral carbon and the attached groups is not random.
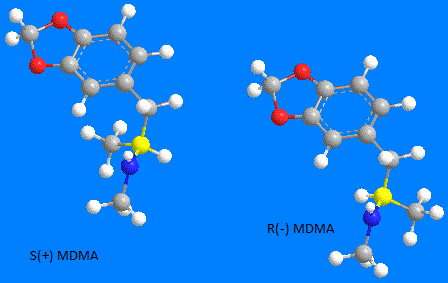
The l and d or (-) and (+) designation is arrived at by by passing polarised light through one pure form. Positions of different groups will effect direction and angle of deflection. Determining this direction assigns a character L or (-) for levorotatory (lefthand ), and D or (+) for dextrorotatory ( righthand )
Another type of isomer configuration, resulting in the assignment of an R or S, is determined by priority placement of the groups attached. Viewing the carbon and attached groups as a tetrahedral with the lowest value group at the top, the order of the other three groups (lowest value to highest value priority) determines R for a right hand circular direction and S for a left.
Note that with many molecules, the R or S assignment may exist in either the (-) or (+) from. However with MDMA the (-) is the R form and the (+) is the S form.
Shulgin states as does the CRC handbook on enantiomers, that the S(+) isomer is the most potent empathy producing form in humans, by quite a degree.
Pihkal #109 MDMA
With MDMA, the usual assignments of activity to optical isomers is reversed from all of the known psychedelic drugs. The more potent isomer is the "S" isomer, which is the more potent form of amphetamine and methamphetamine. This was one of the first clear distinctions that was apparent between MDMA and the structurally related psychedelics (where the "R" isomers are the more active).
Most MDMA is (assumed to be) made from the ketone, an achiral group with a double bond to oxygen [ >C=O ] and planer in form. The N (amine)group is attached to C2 via a process known as reductive amination, givng [ >CH-NH-CH3 ]. Several variations of this reaction exist, using different reducing agents or catalysts.
Other methods that arrive at the target via different mechanisms produce a single enantiomer. Isomers can be separated by chromatography and with some enantiomer resolving agents, but it is unlikely this would be done with street intended product.
As to 32 different forms - I would think - not isomers. And although isomers possess different properties, if it ain't an isomer of MDMA it ain't MDMA. It is definitely possible there could be any of 32 different compounds (more even) in pills. Most likely things are binders, substitutes, unreacted precursors, and products of bad lab routine or side reactions occurring during the synthesis.
What you may be thinking of is isosafrole which can exist as two isomers, cis isosafrole and trans isosafrole. This form of isomer results from substitution across a double bond. Basically it may stick out left or right in direction. If the other end substitute is orientated in the same direction, the molecule is said to be the cis isomer. If in the opposite position it is known as the trans isomer
When the isomerisation is done to safrole both isomers are produced, but the trans isomer is more stable so is the dominant component (70:30).
From here on, whether the iso used is one isomer or the other becomes irrelevant as conversion to the ketone also produces a planer molecule which only exists in one form.
When the final reductive amination occurs, 2 isomers are produced; the (+) or d or -in this case- the S isomer, and the (-) or l or R in a 50:50 ratio.
This route, or a variation of, is the usual method used to produce MDMA. While routes and methods exist for producing a single isomer of MDMA, this is hardly ever done IMO as it would be far from economically justifiable and the market, given the choice, would prefer the 50:50 mixture anyway.
I've been meaning to mention marquis, for a bit. Old pills used to turn purple, then ended up a very dark purple. They didn't go straight to jet-black (actually, I do seem to recall MDA turning the test black). It's been mentioned a couple of times, by others, but no explanation has been given. Surely, if the marquis is reacting differently, it's just further evidence that the new stuff isn't the same.