-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
🌟🌟 Social 🌟🌟 Rectify's molecular poetry thread
- Thread starter Dresden
- Start date
MedicinalUser247
Music Ambassador
- Joined
- Aug 2, 2023
- Messages
- 4,747
Got another cool structure to share with you. The chemical structure is 3,4-TrioxolaneMethamphetamine.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,986
Too unstable, but I can't tell what you mean exactly without a structural diagram.

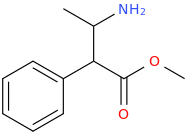
5-OXO-PRELUDIN
1-aza-2-methyl-3-phenyl-4-oxa-5-oxocyclohexane
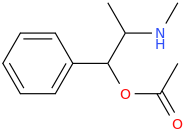
O-ACETYL-METHAMPHETAMINE
1-phenyl-1-acetoxy-2-methylaminopropane
from ephedrine (I think. No idea here on how to protect and then deprotect the amine without hydrolyzing the benzylic OAc, or if N-protection is even necessary in this example. If not, just add some acetic anhydride and BAM!)
OAM, verdict: feels like relatively strong, smooth meth, but nothing like BOUNCE, its reverse ester, which is marvelous.

5-OXO-PRELUDIN
1-aza-2-methyl-3-phenyl-4-oxa-5-oxocyclohexane

O-ACETYL-METHAMPHETAMINE
1-phenyl-1-acetoxy-2-methylaminopropane
from ephedrine (I think. No idea here on how to protect and then deprotect the amine without hydrolyzing the benzylic OAc, or if N-protection is even necessary in this example. If not, just add some acetic anhydride and BAM!)
OAM, verdict: feels like relatively strong, smooth meth, but nothing like BOUNCE, its reverse ester, which is marvelous.
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,986
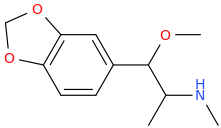
BACARDI_BAT
1-(3,4-methylenedioxyphenyl)-1-methoxy-2-methylaminopropane
I actually had some good pink MDMA Barcardi bats in like 2002.
Outline:
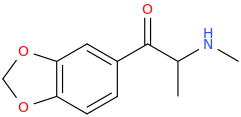
METHYLONE (pretty crappy)
1-(3,4-methylenedioxyphenyl)-1-oxo-2-methylaminopropane
then
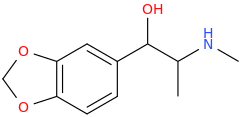
1-(3,4-methylenedioxyphenyl)-1-hydroxy-2-methylaminopropane
Then the NaOMe Williamson Ether Synthesis And Viola!
No harsh, acid catalyzed nitrile oxidation step necessary.
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,986

MYRISTICIN
1-allyl-3,4-methylenedioxy-5-methoxybenzene
KOH, alcohol, heat, reflux

ISOMYRISTICIN
1-propenyl-3,4-methylenedioxy-5-methoxybenzene
Ozonolysis (Zn, HCl, O3+)

MYRISTICINALDEHYDE
1-(oxomethyl)-3,4-methylenedioxy-5-methoxybenzene
an alkyl-amine (such as 1-aminocyclohexane or even CH3NH2) plus CH3CH2NO2 in CH3OH

1-(3,4-methylenedioxy-5-methoxyphenyl)-2-nitro-prop-1-ene
NaBH4, alcohol

1-(3,4-methylenedioxy-5-methoxyphenyl)-2-nitro-propane
Sn/HCl, reflux

THE_LEGENDARY_MMDA
1-(3,4-methylenedioxy-5-methoxyphenyl)-2-aminopropane
said to work best when combined with
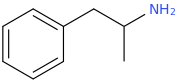
AMP
1-phenyl-2-aminopropane
Since 1887 by Lazaru.
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,986
I just had this idea which may or may not be correct. If fastandbulbous reads this, maybe he could tell us.
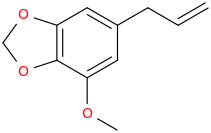
MYRISTICIN
3,4-methylenedioxy-5-methoxy-1-allylbenzene
HBr(g) bubbled through THF

1-(3,4-methylenedioxy-5-methoxy-phenyl)-2-bromopropane
then sodium azide, NaN3

1-(3,4-methylenedioxy-5-methoxy-phenyl)-2-azidopropane
then NaBH4 in alcohol

MYRISTICIN
3,4-methylenedioxy-5-methoxy-1-allylbenzene
HBr(g) bubbled through THF

1-(3,4-methylenedioxy-5-methoxy-phenyl)-2-bromopropane
then sodium azide, NaN3

1-(3,4-methylenedioxy-5-methoxy-phenyl)-2-azidopropane
then NaBH4 in alcohol
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,986
How to make ephedrine or ethylephedrine from benzene: benzene + CH3CH2(C=O)-Cl + AlCl3 -->

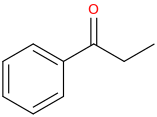
1-phenyl-1-oxopropane + Br2

1-phenyl-1-oxo-2-bromopropane + NH2CH3 (for ephedrine) or NH2CH2CH3 for ethylephedrine

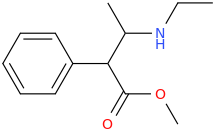
METHCATHINONE (jittery)
1-phenyl-1-oxo-2-methylaminopropane
or
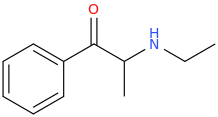
ETHYLONE (weak)
1-phenyl-1-oxo-2-ethylaminopropane
then NaBH4
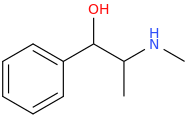
EPHEDRINE
1-phenyl-1-hydroxy-2-methylaminopropane
or
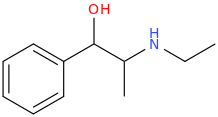
ETHYLEPHEDRINE
1-phenyl-1-hydroxy-2-ethylaminopropane
Both are incredibly valuable precursors for METH, ETH, BOUNCE, and POUNCE.


1-phenyl-1-oxopropane + Br2

1-phenyl-1-oxo-2-bromopropane + NH2CH3 (for ephedrine) or NH2CH2CH3 for ethylephedrine

METHCATHINONE (jittery)
1-phenyl-1-oxo-2-methylaminopropane
or

ETHYLONE (weak)
1-phenyl-1-oxo-2-ethylaminopropane
then NaBH4

EPHEDRINE
1-phenyl-1-hydroxy-2-methylaminopropane
or

ETHYLEPHEDRINE
1-phenyl-1-hydroxy-2-ethylaminopropane
Both are incredibly valuable precursors for METH, ETH, BOUNCE, and POUNCE.
Last edited:
MedicinalUser247
Music Ambassador
- Joined
- Aug 2, 2023
- Messages
- 4,747
Well,... Can you draw a picture of 3,4-TrioxolaneMethamphetamine ?
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,986
How to make propofol [FRANK]:

phenol + HNO3/H2SO4 -->

4-nitrophenol + 2 equivalents
CH3CH2CH2-Cl + AlCl3 -->

2,6-di-isopropyl-4-nitrophenol + Sn/HCl -->

2,6-di-isopropyl-4-aminophenol + CuCl / NaN2 -->

2,6-di-isopropyl-4-diazophenol + H20 -->

FRANK (propofol)
2,6-di-isopropyl-phenol
That's the gist of it anyway. I may have gotten some of the reagents wrong.

phenol + HNO3/H2SO4 -->

4-nitrophenol + 2 equivalents
CH3CH2CH2-Cl + AlCl3 -->

2,6-di-isopropyl-4-nitrophenol + Sn/HCl -->

2,6-di-isopropyl-4-aminophenol + CuCl / NaN2 -->

2,6-di-isopropyl-4-diazophenol + H20 -->

FRANK (propofol)
2,6-di-isopropyl-phenol
That's the gist of it anyway. I may have gotten some of the reagents wrong.
Last edited:
MedicinalUser247
Music Ambassador
- Joined
- Aug 2, 2023
- Messages
- 4,747
It's where the Methylenedioxy has an extra "O" in the middle. So, instead of two O's there's three.