Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
very interesting, like the well know case of Thalidomide
That is my fear.
It turns out that while we knew nitrazepam, nimetazepam, flunitrazepam & clonazepam could cause liver damage, it turns out that nitrazolam and flunitrazolam are just as toxic with reports of severe liver injury.
But the thing is that it's been known since the 1970s that nitrobenzodiazepines were more toxic than other benzodiazepines, it took until 2017 for someone to identify the specific metabolite. I turned out to be:
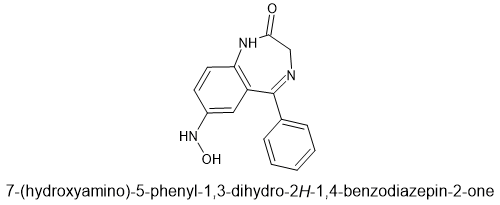
Now, you can see that it doesn't matter about the rest of the scaffold. That 7-nitro (or 8-nitro for 4-ring benzos), their is an enzyme system that WILL reduce that -NO2 to an NH2. The hydroxylation (oxidation of the amine) is actually minor REDUCTION so we are not considering large amounts of this species.
But it can and has killed - a LOT.
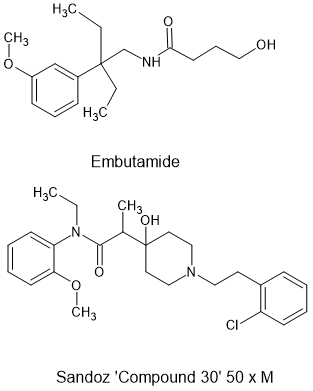
That is why I stick to the modification of known opioids and only introduce modifications as and when needed .Even then I stick to modifications which have a VERY proscribed metabolic pathway.
As an example, mephedrone is VERY safe because that 4-methyl (p-methyl) represents a really good metabolic pathway. Almost 100% of mephedrone undergoes oxidation at that point. First to the aldehyde (via non-specific blood enzymes) and then to the carboxylic acid which will readily form addition salts and be eliminated...
That's why when people place a halogen or pseudohalogen at the para (4) position of the benzene, their will be 8 or 9 minor metabolic pathways and who knows if one of them produces a toxin?
Thalidomide was a really subtle one. Originally the patent called for the (S) isomer (the active enantiomer) but it turned out that the human body inverted that chirality ant it's the (R) isomer that turns out to produce intercalation (insertion into the DNA during cell multiplication). If that sounds horrific, it IS.
So a very good point to make.
IF I need a ring-substitution, methyl is the first one I look at. Then maybe a longer alkyl, methoxy or thiomethoxy BECAUSE the body can metabolize them. If you place a halogen of pseudohalogen then the body cannot alter it. The presence of that (pseudo)halogen also means the body cannot oxidize the ring at the carbons adjacent to that group... so as an example, if you add a p-Br, the entire ring cannot undergo metabolism.
That is why I designed over 50 novel compounds in 2 years an in total only 7 reached market. And people in drug development would be pretty impressed because a 20% lead to produce is REALLY hgh.
I think you might enjoy looking at Paul Janssen's work.

Janssen Pharmaceuticals - Wikipedia
R5 then R79 then R252 then R516. Do you see a pattern? Initially a few dozen compounds would find a product but R4263 (fentanyl) and R33800 (sufentanil) are chemically quite close... but someone made THOUSANDS of compounds to find 1 lead that led to a product.
I think Janssen insisted on a systematic search because the discovery of dextromoramide (R875) is an 'island of activity' i.e. even very closely related compounds had little or no activity. I don't know if his team used rational design, but to me it represents one of the most impressive discoveries ever.