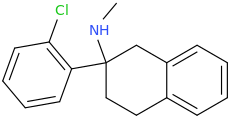
Spiroxetamine..
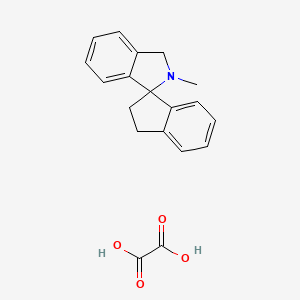
12x more potent than ketamine (IC50 0.065uM v 0.86uM.. https://pubchem.ncbi.nlm.nih.gov/assay/bioactivity.html?cid=44370217 Add a methoxy somewhere?or a Halo? look pretty easy straightforward synthesis https://pubchem.ncbi.nlm.nih.gov/compound/44370217#section=Top
12x more potent than ketamine (IC50 0.065uM v 0.86uM.. https://pubchem.ncbi.nlm.nih.gov/assay/bioactivity.html?cid=44370217 Add a methoxy somewhere?or a Halo? look pretty easy straightforward synthesis https://pubchem.ncbi.nlm.nih.gov/compound/44370217#section=Top