Nagelfar
Bluelight Crew
What would this be like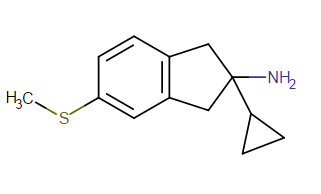
Looks like it would be a dopamine agonist like Pramipexole or a serotonin agonist, or maybe just inactive. But those are my best guesses
N&PD Moderators: Skorpio | someguyontheinternet
What would this be like
It'll migrate over to the nitrogen, form an imine, and be hydrolyzed to a ketone by water.
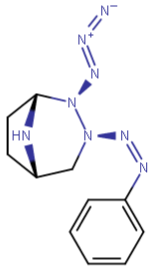
Give me a stability lesson with this; which nitrogens are possible with it still being stable:

I am not sure if I get what you saying but I have the impression you may be talking enamines which are indeed not stable in water and will get hydrolyzed quickly to the corresponding ketone and amines. In enamines, the N is connected directly to the sp2 carbon to give vinyl amines which are not stable. But these are allyl amines(not vinyl).. they should be fine. Unless there is something specific about these particular compounds!It'll migrate over to the nitrogen, form an imine, and be hydrolyzed to a ketone by water.
Title: Characterization of a series of 3-amino-2-phenylpropene derivatives as novel bovine chromaffin vesicular monoamine transporter inhibitors.
Abstract: A series of 3-amino-2-phenylpropene (APP) derivatives have been synthesized and characterized as novel competitive inhibitors, with K(i) values in the microM range, for the bovine chromaffin granule membrane monoamine transporter(s) (bVMAT). Although, these inhibitors are structurally similar to the bVMAT substrate tyramine, none of them were measurably transported into the granule. Structure-activity studies have revealed that, while the 3'- or 4'-OH groups on the aromatic ring enhance the inhibition potency, Me or OMe groups in these positions reduce the inhibition potency. Halogen substitution on the 4'-position of the aromatic ring causes gradual increase of the inhibition potency parallel to the electron donor ability of the halogen. Substituents on the NH(2) as well as on the 3-position of the alkyl chain reduce the inhibition potency. Comparative structure-activity analyses of APP derivatives with tyramine and the neurotoxin 1-methyl-4-phenylpyridinium suggest that the flexibility of the side chain and the relative orientation of the NH(2) group may be critical for the efficient transport of the substrate through the bVMAT. Comparable bVMAT affinities of these inhibitors to that of DA and other pharmacologically active amines suggest that they are suitable for the structure-activity and mechanistic studies of monoamine transporters and may also be useful in modeling the mechanism of action of amphetamine-related derivatives. https://pubchem.ncbi.nlm.nih.gov/bioassay/216244#section=Top
the carbon connected directly to the amine is sp3. the double bond cannot go there.
has anyone already thought of this one? https://imgur.com/gallery/81Ggg
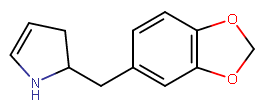
I am not sure that I follow... you mean that the benzylic carbon will be protonated I guess? but how will that turn the sp3 carbon at the 2-position if the butane chain into sp2. can you clarify the mechanism?double bond can migrate there in acidic condition, styrene-type structure is very easy to get protonated,
after protonation and de-protonation, db has 2 site to go, one to original, other to the enamine.