Incunabula
Bluelighter
- Joined
- Dec 10, 2010
- Messages
- 1,861
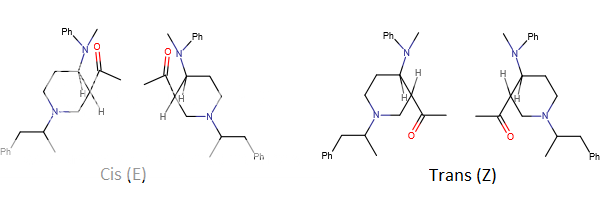
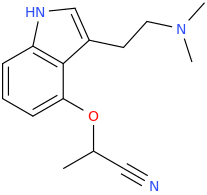
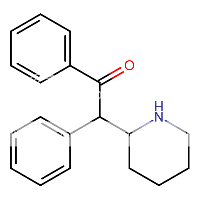
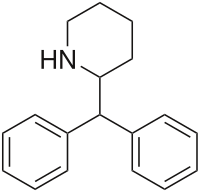
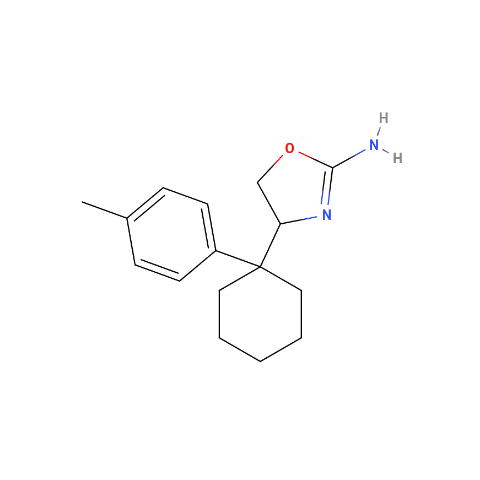
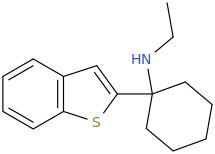
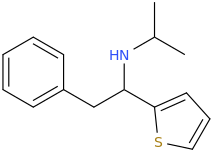
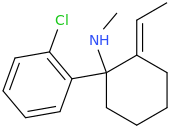
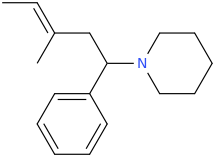
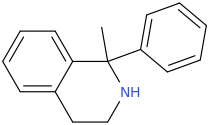
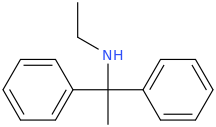
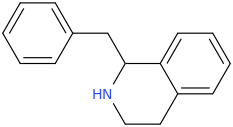
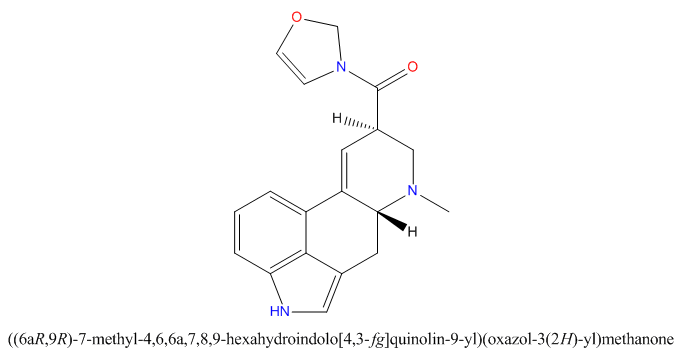
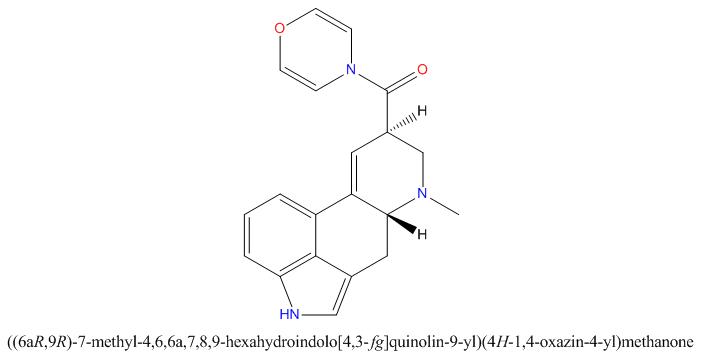
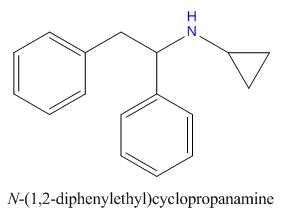
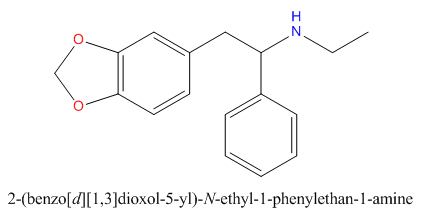
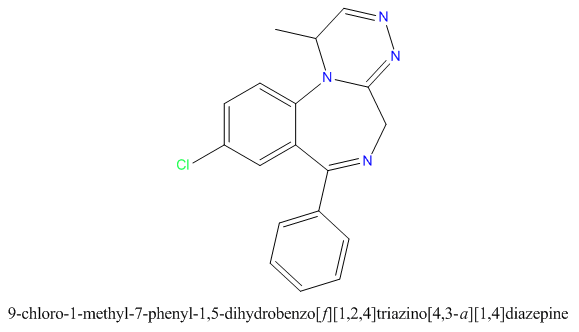
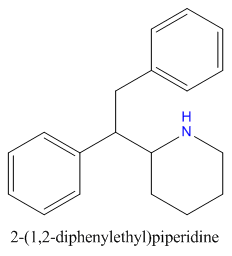
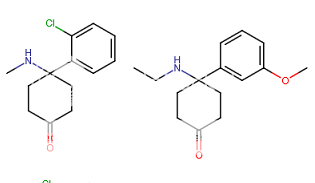
when there are a pair of chiral centers next to each other, there is a cis/trans or erythro/threo/meso relationship
you are correct about the swirly lines meaning the bonds can either stick out front or behind the paper. however only sp3 carbon bonds that are unconstrained can rotate - like the bond between the two carbons in ethane. if it's constrained in a ring then bond rotation would invert or pucker the ring and there can be a singificant energy barrier to that.
Thanks, I get it now