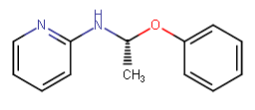
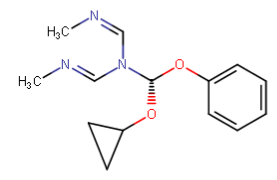
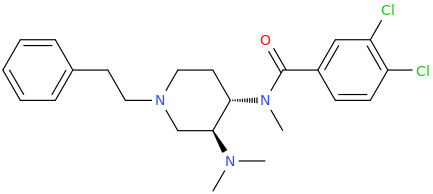
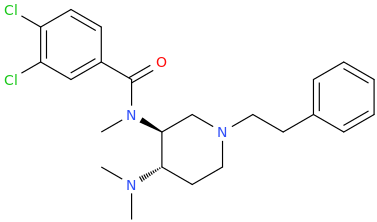
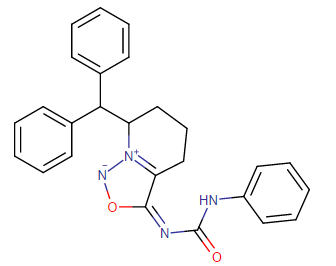
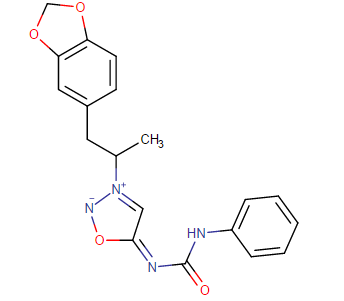
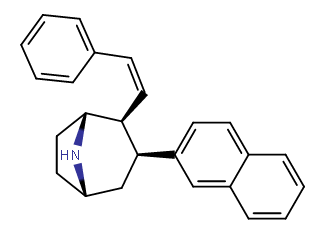
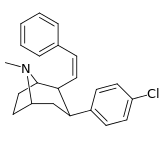
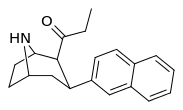
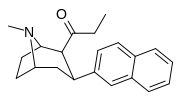
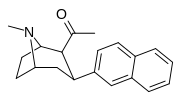
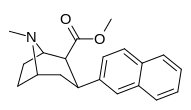
Nagelfar
Bluelight Crew
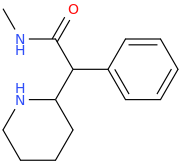
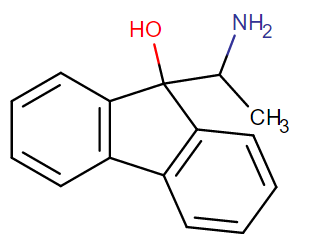
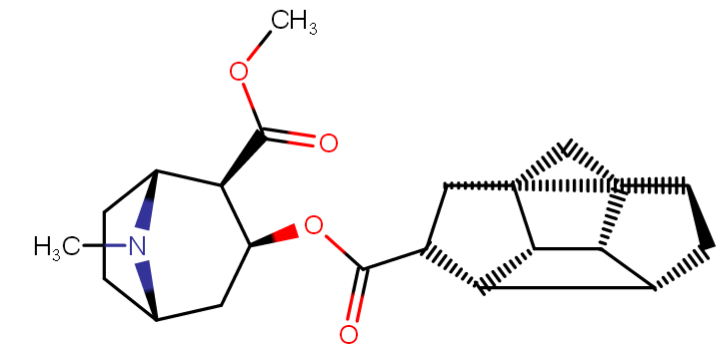
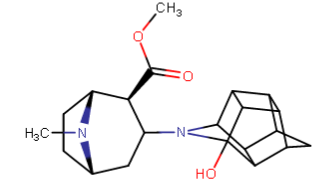
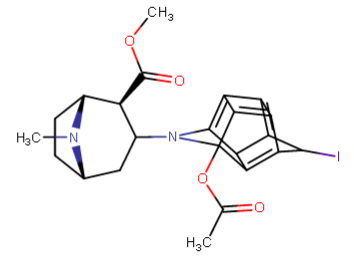
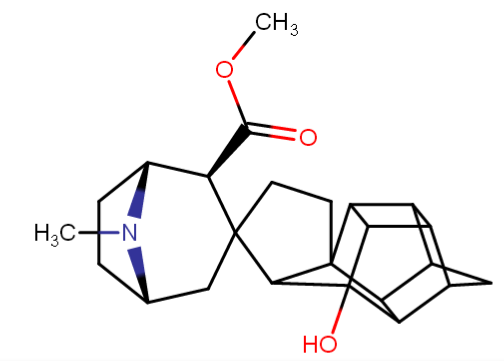
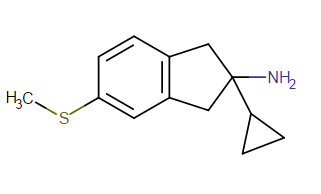
^anyhow, I am believing the above would have anti-chlorinergic effects, like tematropium & tropatepine, possibly, but the cyclopentane is smaller so perhaps not.
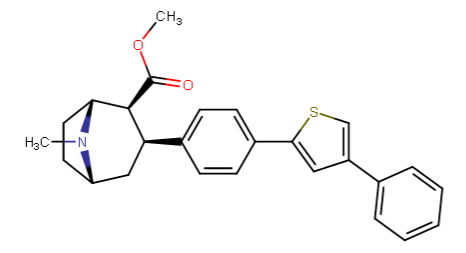
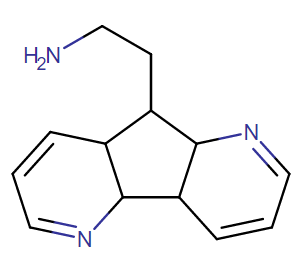
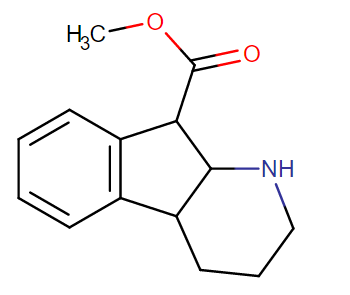
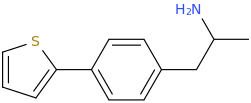
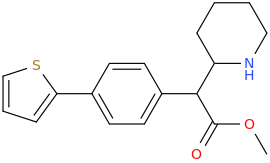
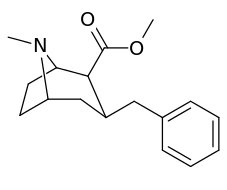
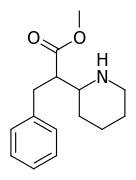
2-Thiophene with the length para addition that is between RTI-430 & RTI-298 (para of benzene equals: –C≡C-CH2Ph) which has the distal extra binding site for dopaminergic affinity.
e.g. between this:
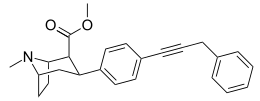
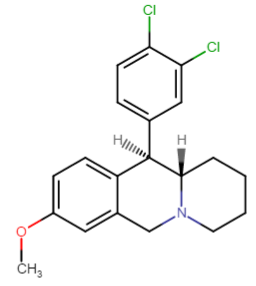
52 ± 12.8 DAT
And this:
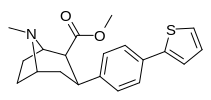
1.82 ± 0.42 DAT

2-Thiophene with the length para addition that is between RTI-430 & RTI-298 (para of benzene equals: –C≡C-CH2Ph) which has the distal extra binding site for dopaminergic affinity.
e.g. between this:

52 ± 12.8 DAT
And this:

1.82 ± 0.42 DAT
Last edited: