-
Select Your Topic Then Scroll Down
Alcohol Bupe Benzos Cocaine Heroin Opioids RCs Stimulants Misc Harm Reduction All Topics Gabapentinoids Tired of your habit? Struggling to cope?
Want to regain control or get sober?
Visit our Recovery Support Forums -
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
RCs Bromazolam
- Thread starter JoEhJoEh
- Start date
Cream Gravy?
Bluelight Crew
- Joined
- Jan 28, 2014
- Messages
- 12,457
I like to take it at 5mg but I'd say 1-2mg is minimum dose.
Is Bromazolam detectable in drug tests? Answer: Yes. I took a saliva test and the substance for which it was tested positive for was Clonazepam. I don't know what the results would've been if I had taken a urine test.
Heres my story:
When I used Bromazolam about a year ago, I had to take this drug test and the procedure went like this: A nurse will give you a yellow-colored like liquid and the taste is repugnant but before they give it to you they want you to wash your mouth with water before you take the yellow liquid orally and then you'll have to wash it in your mouth just like any other mouthwash for atleast 2 minutes. Then, you spit it into a cup and they'll send it to a lab and analyze it.
About a week later the results came back and I tested positive for Clonazepam (which goes by the brand name Klonopin in the U.S). I was surprised because I have never ever taken Clonazepam in my life.
Heres my story:
When I used Bromazolam about a year ago, I had to take this drug test and the procedure went like this: A nurse will give you a yellow-colored like liquid and the taste is repugnant but before they give it to you they want you to wash your mouth with water before you take the yellow liquid orally and then you'll have to wash it in your mouth just like any other mouthwash for atleast 2 minutes. Then, you spit it into a cup and they'll send it to a lab and analyze it.
About a week later the results came back and I tested positive for Clonazepam (which goes by the brand name Klonopin in the U.S). I was surprised because I have never ever taken Clonazepam in my life.
emkee_reinvented
Bluelighter
Is there any conclusive info on the dosage of Bromazolam?
They are supplied in 3 mg pellet's and I am planning on taking 1.5 mg ( a half pellet) tonight. Which might be to little but that's okay as I should be tapering anyway. My tolerance is naturally high. But on average I'll take 1 mg Etizolam and recently 1 mg Flu-Etizolam. At night.
My hope is that it is just a bit more hypnotic as the two benzo's mentioned enabling me to lower my dose.
And get back to sober sleep, which is also a bit troublesome as they detected a sleeping dis-order. I have way too little deep sleep.
They are supplied in 3 mg pellet's and I am planning on taking 1.5 mg ( a half pellet) tonight. Which might be to little but that's okay as I should be tapering anyway. My tolerance is naturally high. But on average I'll take 1 mg Etizolam and recently 1 mg Flu-Etizolam. At night.
My hope is that it is just a bit more hypnotic as the two benzo's mentioned enabling me to lower my dose.
And get back to sober sleep, which is also a bit troublesome as they detected a sleeping dis-order. I have way too little deep sleep.
There is not much information or documentation to be found regarding Bromazolam unfortunately. Even the half-life of Bromazolam remains a mystery. Not all, but many have RC-Benzos have had their half-life discovered and documented such as Diclazepam, which has a half-life of at least 42 hours. Source: https://psychonautwiki.org/wiki/DiclazepamIs there any conclusive info on the dosage of Bromazolam?
They are supplied in 3 mg pellet's and I am planning on taking 1.5 mg ( a half pellet) tonight. Which might be to little but that's okay as I should be tapering anyway. My tolerance is naturally high. But on average I'll take 1 mg Etizolam and recently 1 mg Flu-Etizolam. At night.
My hope is that it is just a bit more hypnotic as the two benzo's mentioned enabling me to lower my dose.
And get back to sober sleep, which is also a bit troublesome as they detected a sleeping dis-order. I have way too little deep sleep.
Bromazolam is very common and sold as 3mg pellets/pills both in NA/EU. And reports from users I've read from a foreign drug forum; Flu-Etizolam is supposedly more hypnotic and sedative than Etizolam.
As for the half-life of Bromazolam, I've read reports that it may have a higher half-life than Alprazolam (Xanax), which I believe is accurate.
I would certainly recommend Etizolam for sleep simply for its therapeutic properties. I haven't had it in years. I prefer Etizolam over Bromazolam but they're hard to come by these days in my area so I just stick to Bromazolam
Last edited:
emkee_reinvented
Bluelighter
My hopes are its not too long. Something in the line of Etizolam would be great.There is not much information or documentation to be found regarding Bromazolam unfortunately. Even the half-life of Bromazolam remains a mystery ....
And a bit more hypnotic. But I'll report back if I have tested em out
Etizolam sadly was banned.I would certainly recommend Etizolam for sleep simply for its therapeutic properties. I haven't had it in years. I prefer Etizolam over Bromazolam but they're hard to come by these days in my area so I just stick to Bromazolam
emkee_reinvented
Bluelighter
As follow up 1.5 mg was to less to induce sleep. So it consisted more of half sleep. At 6:00 o'clock I regained consciousness and re dosed the bigger half of the pellet.
They are unbreakable so my first dosage was maximum 1mg with 0.5 mg Flu-Etizolam added. After re dosing the remaining pellet I relaxed and slept another 4 hour's waking up more refreshed then when not taking a Benzo. No lingering effects so its half live is not absurd like Diazepam, Diclazepam and a few others.
So far it seems a reasonable Benzo. Maybe a bit better then Alprazolam as that one made me sad.
edit: smoked some Weed a minute ago which seemed a bit diff. So there seem to be some lingering effect's of the dose I took 9.5 hours ago.
They are unbreakable so my first dosage was maximum 1mg with 0.5 mg Flu-Etizolam added. After re dosing the remaining pellet I relaxed and slept another 4 hour's waking up more refreshed then when not taking a Benzo. No lingering effects so its half live is not absurd like Diazepam, Diclazepam and a few others.
So far it seems a reasonable Benzo. Maybe a bit better then Alprazolam as that one made me sad.
edit: smoked some Weed a minute ago which seemed a bit diff. So there seem to be some lingering effect's of the dose I took 9.5 hours ago.
Last edited:
emkee_reinvented
Bluelighter
3 mg also linger's so the half live is probably longer then Etizolam.
It also felt somewhat stronger then 1 mg Etizolam.
It also felt somewhat stronger then 1 mg Etizolam.
unodelacosa
Bluelighter
Bromazolam only differs from Alprazolam (Xanax) by substituting a bromine atom where there is normally a chlorine atom. Bromine and chlorine are both in the same column on the periodic table, both halogens with the same arrangement of electrons in their outer valence shell. Fluorine is the lightest halogen followed by chlorine, bromine, and then iodine. I've found that Bromazolam is mostly similar to Alprazolam in effect, except that for me bromazolam brings more anterograde amnesia and feels heavier in qualitative terms.3 mg also linger's so the half live is probably longer then Etizolam.
It also felt somewhat stronger then 1 mg Etizolam.
Xorkoth
Bluelight Crew
Bromazolam is similar to alprazolam but more sedating, a a little less potent. It lasts longer than etizolam and produces more rebound anxiety and dependence. It's around the same potency as etizolam in my experience.
unodelacosa
Bluelighter
Yes Bromazolam definitely lasts longer than Alprazolam and it slaps harder, which aren't good things when you're seeking a 'Tiz replacement. Similarly, Flubromazepam is a bit too heavy and definitely has too long of a half-life to elimination as does the compound formed when fusing shut that triazolo ring a la triazolam & alprazolam, Flubromazolam. Heavy hitters. In the medium-weight division is clonazolam, which is hella potent, but not as heavy nor as long-lasting as the aforementioned benzos. I tend to rank that one pretty highly.3 mg also linger's so the half live is probably longer then Etizolam.
It also felt somewhat stronger then 1 mg Etizolam.
I believe the popularity of benzos, according to prescriptions, goes like this:
#1 Alprazolam (Xanax) – a Triazolo benzo
#2 Clonazepam (Klonopin) – a 7-nitro benzo
#3 Lorazepam (Ativan) – a 3-hydroxy benzo
#4 Diazepam (Valium) – a 2-keto benzo
I think that holds up pretty true.
Qualitatively, it's stronger than Etizolam, but mg for mg, Etiz seems more potent. I'll take 1 - 2 mg of etiz usually whereas it's more like 3 -5 mg of bromazolam for me.It's around the same potency as etizolam in my experience.
Cream Gravy?
Bluelight Crew
- Joined
- Jan 28, 2014
- Messages
- 12,457
My thoughts exactly. Heavier and more amnesic.I've found that Bromazolam is mostly similar to Alprazolam in effect, except that for me bromazolam brings more anterograde amnesia and feels heavier in qualitative terms.
emkee_reinvented
Bluelighter
My list would def be diff.Yes Bromazolam definitely lasts longer than Alprazolam and it slaps harder, which aren't good things when you're seeking a 'Tiz replacement. Similarly, Flubromazepam is a bit too heavy and definitely has too long of a half-life to elimination as does the compound formed when fusing shut that triazolo ring a la triazolam & alprazolam, Flubromazolam. Heavy hitters. In the medium-weight division is clonazolam, which is hella potent, but not as heavy nor as long-lasting as the aforementioned benzos. I tend to rank that one pretty highly.
I believe the popularity of benzos, according to prescriptions, goes like this:
#1 Alprazolam (Xanax) – a Triazolo benzo
#2 Clonazepam (Klonopin) – a 7-nitro benzo
#3 Lorazepam (Ativan) – a 3-hydroxy benzo
#4 Diazepam (Valium) – a 2-keto benzo
I think that holds up pretty true.
Qualitatively, it's stronger than Etizolam, but mg for mg, Etiz seems more potent. I'll take 1 - 2 mg of etiz usually whereas it's more like 3 -5 mg of bromazolam for me.
Diazepam superior on all fields (muscles, anxiety, sedation and anti-convulsant), only the duration is way to long. And I miss Temazepam in the ranking easily the most recreative benzo ime. And Alprazolam was not that special, I think i like 2,3 and 4 more.
unodelacosa
Bluelighter
So, I’m going off memory having recently seen these stats. To me, it also seems like the preferential order for the majority of benzo-users I know.My list would def be diff.
Diazepam superior on all fields (muscles, anxiety, sedation and anti-convulsant), only the duration is way to long. And I miss Temazepam in the ranking easily the most recreative benzo ime. And Alprazolam was not that special, I think i like 2,3 and 4 more.
And to me, Alprazolam has superior anxiolysis to Diazepam, but it’s better suited for treatment of acute onsets of panic disorder, whereas Valium tends to have a longer-lasting period of lowered panic reactions instead of full-on attacks. It’s nearly impossible for me to panic when I’m on Alprazolam, meanwhile Diazepam lasts kinda long and tends to leave me sluggish the next day while still subject to some anxiety.
For anyone with acute panic attacks, it’s plainly obvious to me why Alprazolam is so preferred, plus the rapid onset of action and the shorter duration and half-life than many other benzos… what’s not to love? So I still rank Alprazolam as one of the very best, and I honestly would rate Etizolam right up there, too in my personal favorite benzos. The fact there’s less build-up in the nucleus accumbens indicates lessened risk of developing both dependence and tolerance, and it’s only partial cross-tolerant with other, actual “benzos” owing to it being a thienotriazolodiazepine technically speaking, what with it’s fancy sparkling sulphur molecule staring us in the face…
Let’s consider benzodiazepines for a moment, shall we? The benzodiazepine core is found in all benzos. There are multiple positions that can be substituted with different substance groups.

- R⁷ is always a halogen (bromine or chlorine) or a nitro group. Bromine is most potent, but also most hypnotic; nitro is most recreational.
- R²' can be nothing or a halogen (chlorine, fluorine). Halogens tend to increase the potency a lot.
- R² is pretty much always a ketone (except for triazolobenzodiazepines, which I'll explain).
- R¹ can be nothing or a methyl group (again, exception triazolobenzodiazepines).
- In triazolobenzodiazepines (such as Alprazolam) R¹ and R² are infused into a 1,2,4-triazole ring.
- On the triazole ring, the methyl group can be removed cutting potency by 50% but increasing duration (see: Estazolam or Metizolam).
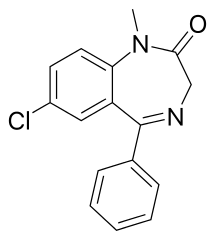
Compare that to Clonazepam (Klonopin):

R⁷ is a nitro group, R²' chlorine. The halogen at R²' gives it a much higher potency compared to Diazepam.
Triazolobenzodiazepines with a R⁷ nitro group tend to be the most euphoric/recreational benzodiazepines.
The name Flu-ni-trazolam tells you all about the structure already:
- Flu = fluorine at R²'
- ni = nitro at R⁷
- triazolam = triazolo group.
Flunitrazolam combines the most recreational substitutions (triazolo and R⁷ nitro) with R²' fluorine, which provides the highest potency. It's Rohypnol (Flunitrazepam) with the triazole ring on it.
Of course there's also a few more possible changes, such as 3-methyl benzodiazepines (which are metabolites of other benzodiazepines), for example Oxazepam, which is a metabolite of Diazepam.

It is also possible to replace the phenyl ring with a pyridine one, as seen in Pyrazolam. This seems to be of similar potency as a 2-chlorophenyl group, but causes the substance to be active at different subreceptors.

Another possibility is going from benzodiazepines to thienodiazepines. Here the upper phenyl ring is replaced by thiophene. This also increases the potency a bit. An example is Etizolam:

But it's not always as it seems. Using the logic above, the most potent possible benzo derivative should be something like Flubrotizolam, and yet Flubromazolam seems more potent.
emkee_reinvented
Bluelighter
In reality Etizolam is about as good as it gets.
Fast working Benzo's seem preferable. But Diazepam has a nice broad spectrum despite not being my favorit, Temazepam works to fast. Its to recreational.
Going the triazolo way Flunitrazolam was more dissapointing then Flunitrazepam regarding its triazolo propereties this was unexpected. But it was just average a mid way. Nothing exceptional. I would place it between Flu-Etizolam which is stronger and flu-Alprazolam which last's longer.
Fast working Benzo's seem preferable. But Diazepam has a nice broad spectrum despite not being my favorit, Temazepam works to fast. Its to recreational.
Going the triazolo way Flunitrazolam was more dissapointing then Flunitrazepam regarding its triazolo propereties this was unexpected. But it was just average a mid way. Nothing exceptional. I would place it between Flu-Etizolam which is stronger and flu-Alprazolam which last's longer.
unodelacosa
Bluelighter
Ok, now let's talk about…
The GABA(A) receptor complex has many different subunits, which in turn have isoforms, otherwise known as subtypes (α1- 6, β1-4, γ1-3, δ, ε, θ, and ρ1-2). Different benzodiazepines (BZD) have different affinities for GABA(A) receptors made up of different collection of subunits, and this means that their pharmacological profile varies with subtype selectivity. Only three of the subunits (α, β, and γ) are benzodiazepine sensitive. The α (alpha) subunit has 6 subtypes, but only α1, α2, α3, and α5 are BZD sensitive. The β (beta) subunit has 4 subtypes, but only the β3 subtype is BZD sensitive. The γ (gamma) subunit has 3 subtypes, but only the γ2 subtype is BZD sensitive.
Subunits from only one class (α) or two classes (α and β) can form functional GABA receptors under experimental conditions, but subunits from three classes (α, β, and γ) are needed for full receptor function. These three subunits also compose most of the GABA(A) receptors in the mammalian brain.
GABA
Benzos work by increasing the efficiency of a natural brain chemical, GABA, to decrease the excitability of neurons. This reduces the communication between neurons and therefore has a calming effect on many of the functions of the brain.The GABA(A) receptor complex has many different subunits, which in turn have isoforms, otherwise known as subtypes (α1- 6, β1-4, γ1-3, δ, ε, θ, and ρ1-2). Different benzodiazepines (BZD) have different affinities for GABA(A) receptors made up of different collection of subunits, and this means that their pharmacological profile varies with subtype selectivity. Only three of the subunits (α, β, and γ) are benzodiazepine sensitive. The α (alpha) subunit has 6 subtypes, but only α1, α2, α3, and α5 are BZD sensitive. The β (beta) subunit has 4 subtypes, but only the β3 subtype is BZD sensitive. The γ (gamma) subunit has 3 subtypes, but only the γ2 subtype is BZD sensitive.
Subunits from only one class (α) or two classes (α and β) can form functional GABA receptors under experimental conditions, but subunits from three classes (α, β, and γ) are needed for full receptor function. These three subunits also compose most of the GABA(A) receptors in the mammalian brain.
The Alpha (α) subunit
The most important subunit is the "alpha" (α) and its subtypes isoforms (α1,2,3,and 5). The alpha subunit is responsible for mediating most of the effects of the benzos. All benzos bind to this subunit but they also all have different affinity levels to the different subtypes.- α1 subtype: Sedation, respiratory depression, sleep, ataxia, motor-impairment, amnesia, anti-convulsive, and reinforcing behavior
- α2 subtype: Anxiolysis, disinhibition
- α3 subtype: Anxiolysis, anti-convulsive, muscle relaxation
- α5 subtype: Learning and memory, amnesia, minor sedation
- α3 & α5 subtype: Sensorimotor information processing
The Beta (β) and Gamma (γ) subunits
- γ2 subtype: Physical dependence, respiratory depression
- β3 subtype: Anti-convulsive, minor sedation, muscle relaxation, various other reactions related to respiration. This receptor subtype is a barbiturate receptor.
Benzos affinity to the GABA(A) receptors
Alpha 1 affinity
- High α1 affinity: midazolam, triazolam, flunitrazepam, temazepam, lormetazepam, nitrazepam, brotizolam, nimetazepam, loprazolam, and flutoprazepam.
- Low to Moderate α1 affinity: wide range of 1,4 benzodiazepines including diazepam, estazolam, flurazepam, oxazepam, lorazepam, alprazolam, bromazepam, camazepam, quazepam (highly selective affinity), clonazepam, medazepam, nordazepam, chlordiazepoxide (very weak affinity), clorazepate, and most other benzo as all benzos are α1 agonists with varying degrees of affinity levels. Also included here are the non-benzodiazepine "z-drugs" such as zolpidem, zaleplon, zopiclone, and eszopiclone which are all highly selective of the α1 subtype receptor but with only weak to moderate affinity.
Alpha 2 affinity
- High α2 affinity: diazepam, clonazepam, bromazepam, lorazepam, alprazolam, camazepam, nitrazepam, loprazolam, lormetazepam, and flutoprazepam.
- Moderate α2 affinity: oxazepam, prazepam, phenazepam, temazepam, flunitrazepam, halazepam, midazolam, and other less commonly known benzos.
- Weak α2 affinity: triazolam, chlordiazepoxide (stronger affinity for α3), brotizolam, quazepam, tetrazepam (stronger affinity for α3), and a few others.
Alpha 3 affinity
- High α3 affinity: diazepam, clonazepam, temazepam, lorazepam, tetrazepam, flunitrazepam, nimetazepam, phenazepam, and bromazepam.
- Moderate α3 affinity: alprazolam, adinazolam, estazolam, chlordiazepoxide, clorazepate, and flurazepam.
Beta (β) and Gamma (γ) affinity
- High γ2 affinity (these benzos are the most physically addictive): temazepam, brotizolam, triazolam, alprazolam, lorazepam, loprazolam, midazolam, flunitrazepam, clonazepam, lormetazepam, flutoprazepam, nitrazepam, nimetazepam, and estazolam
- Low to moderate γ2 affinity: diazepam, chlordiazepoxide, oxazepam, and most other benzos.
- High β3 affinity: mostly the hypnotics (nitrazepam, temazepam, triazolam, etc)
Last edited:
Temazepam is actually a very short-acting benzodiazepine. The traditional "8-20hrs" claimed by the makers of Restoril, back in 1981 by Mallinckrodt. The half-life is actually 4-10 hrs. With a very rapid onset. Restoril is the worst temazepam formulation. The temazepam tablets and gelcaps you might find in Europe and Australia are the best.For sure. It’s the only common intermediate acting hypnotic benzo you’ll find. In the U.S. anyway. Evidently it’s the fifth most commonly prescribed benzo here.
Yeah I don’t like the crazy long half life of diazepam and the way it goes and goes all the way through the following day leaving me sluggish and sedate, both mentally and physically. But it’s the only benzo that by itself is euphoric for me for about 3-4 hrs after dosing. Years back I bought a bottle of 100 Roche brand name 10mg Valium and would take 30-40mg every Friday evening after school and work was finished for the week. They were shockingly superior to all the other generic versions I’ve ever had. God those were some pleasant nights.
Wouldn't a chlorine atom increase the potency slightly vs. a bromine atom? I know a fluorine tends to increase potency by a significant margin. For example, flunitrazepam is fluorinated nitrazepam, increasing potency by weight (10x as 1mg flunitrazepam = `10mg nitrazepam). Aside from alprazolam, flunitrazepam is the most overrated benzo - why? No clue). Nitrazepam has a more rapid onset, lasts longer (not always a good), has a similar feel to flunitrazepam, but in my experience, nitrazepam over flunitrazepam anyday.Bromazolam only differs from Alprazolam (Xanax) by substituting a bromine atom where there is normally a chlorine atom. Bromine and chlorine are both in the same column on the periodic table, both halogens with the same arrangement of electrons in their outer valence shell. Fluorine is the lightest halogen followed by chlorine, bromine, and then iodine. I've found that Bromazolam is mostly similar to Alprazolam in effect, except that for me bromazolam brings more anterograde amnesia and feels heavier in qualitative terms.
Wouldn't a chlorine atom increase the potency slightly vs. a bromine atom? I know a fluorine tends to increase potency by a significant margin. For example, flunitrazepam is fluorinated nitrazepam, increasing potency by weight (10x as 1mg flunitrazepam = `10mg nitrazepam). Aside from alprazolam, flunitrazepam is the most overrated benzo - why? No clue). Nitrazepam has a more rapid onset, lasts longer (not always a good), has a similar feel to flunitrazepam, but in my experience, nitrazepam over flunitrazepam anyday.
Yes chlorine would increase potency relative bromine. As such bromazolam is slightly less potent than alprazolam, with alprazolam being around 1.25x the potency of bromazolam. Bromazolam is clearly more hypnotic and less anxiolytic however.
If alprazolam was an RC and not a pharmaceutical we'd call it clomazolam (by these naming conventions). Or maybe deschlorotriazolam, though i suppose that doesn't specify where the missing chlorine is. Likewise i suppose you could call triazolam, "cloalprazolam". Or even better, if both alprazolam and triazolam were RC benzos, by the current naming conventions one would could call triazolam "cloclomazolam" which has a nice ring to ring to (and sort of descriptive of it's effect
I agree with etizolam being the best of the RC benzos. Closest to alprazolam, which I personally consider the most reinforcing and enjoyable benzo. Temazepam is good if you are looking for the physically euphoric aspect of benzos, but the headspace of alprazolam is quite upbeat, though ultimately a trainwreck. All benzos are trainwrecks ultimately. The liberating feeling of the more stimulating benzos (like alprazolam and etizolam) is amazing, but it becomes too liberating, sort of cuts the brake cables for me.
Xorkoth
Bluelight Crew
I find etizolam quite sedating, to the point I have a hard time staying awake even on 1mg. I only use it for sleep so that's good for me, but wouldn't ever consider it stimulating.



