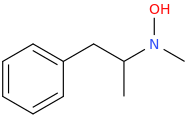
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
🌟🌟 Social 🌟🌟 Rectify's molecular poetry thread
- Thread starter Dresden
- Start date
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 511
It is commerically available: https://www.manchesterorganics.com/F16112
4DQSAR
Bluelighter
- Joined
- Feb 3, 2025
- Messages
- 1,361
Mate - I don't touch stimulants beyond caffeine. I'm quite capable of being anxious without chemicals. In fact, I don't consume anything not prescribed and I don't even like HAVING to take those.
I also find the chemistry sort of dull. Once you have seen a few clandestine labs, you quickly decide that no, you don't want to put ANYTHING made that way into your body.
I like opioid chemistry BECAUSE nobody has been able to find a set of rules. It's a genuine unknown where an independant researcher can genuinely improve upon that which is known. But I don't go anywhere NEAR breaking the law, ever.
I also find the chemistry sort of dull. Once you have seen a few clandestine labs, you quickly decide that no, you don't want to put ANYTHING made that way into your body.
I like opioid chemistry BECAUSE nobody has been able to find a set of rules. It's a genuine unknown where an independant researcher can genuinely improve upon that which is known. But I don't go anywhere NEAR breaking the law, ever.
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 511
This is just analogs anyway you need the original Bruce Horrom patent i cited above.

 pubchem.ncbi.nlm.nih.gov
pubchem.ncbi.nlm.nih.gov

 pubchem.ncbi.nlm.nih.gov
but the compound you called Snoopy (i called it Triflutamine or Trifluorex) looks a most interesting compound to study.
pubchem.ncbi.nlm.nih.gov
but the compound you called Snoopy (i called it Triflutamine or Trifluorex) looks a most interesting compound to study.
1-[4-Fluoro-3-(trifluoromethyl)phenyl]propan-2-amine
1-[4-Fluoro-3-(trifluoromethyl)phenyl]propan-2-amine | C10H11F4N | CID 79020960 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
1-[3-Fluoro-4-(trifluoromethyl)phenyl]propan-2-amine
1-[3-Fluoro-4-(trifluoromethyl)phenyl]propan-2-amine | C10H11F4N | CID 103694471 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 511
I found a new drug called IDDC that looks interesting:

Eckard Weber, John Keana, & Peter Barmettler, WO1990012575 (Oregon State Board of Higher Education, Oregon State).
Gee KR, Barmettler P, Rhodes MR, McBurney RN, Reddy NL, Hu LY, Cotter RE, Hamilton PN, Weber E, Keana JF. 10,5-(Iminomethano)-10,11-dihydro-5H-dibenzo[a,d]cycloheptene and derivatives. Potent PCP receptor ligands. J Med Chem. 1993 Jul 9;36(14):1938-46. doi: 10.1021/jm00066a002. PMID: 8101572.
It shares the same precursor that is used to make Diphenidine, namely 1,2-diphenylethylamine.

Eckard Weber, John Keana, & Peter Barmettler, WO1990012575 (Oregon State Board of Higher Education, Oregon State).
Gee KR, Barmettler P, Rhodes MR, McBurney RN, Reddy NL, Hu LY, Cotter RE, Hamilton PN, Weber E, Keana JF. 10,5-(Iminomethano)-10,11-dihydro-5H-dibenzo[a,d]cycloheptene and derivatives. Potent PCP receptor ligands. J Med Chem. 1993 Jul 9;36(14):1938-46. doi: 10.1021/jm00066a002. PMID: 8101572.
It shares the same precursor that is used to make Diphenidine, namely 1,2-diphenylethylamine.
4DQSAR
Bluelighter
- Joined
- Feb 3, 2025
- Messages
- 1,361
Well, isophenidine was the much better product. Bu as stated, the IDIOTS in the lab bought something like 1000l of piperidine so we VERY keen that dipenidine analogues were the way.
They EVEN had the cheek to say 'oh, we can't make the N-isopropyl because of the low BP of the amine'. So I sent a RT route with quantitative yields and methanol as solvent... and they STILL tried to tell the boss they couldn't make it...
Funny - the boss sacked them quite soon afterwards. If I take a man's dollar, I work to make the man money. The labrats were only interested in their own cut. Understandable - But don't take a banana to a knife-fight if you intend to argue facile syntheses.
I ASSUME these compounds to be more potent - but I can make isophendine for about £400/Kg - see also 'very facile indeed'.
They EVEN had the cheek to say 'oh, we can't make the N-isopropyl because of the low BP of the amine'. So I sent a RT route with quantitative yields and methanol as solvent... and they STILL tried to tell the boss they couldn't make it...
Funny - the boss sacked them quite soon afterwards. If I take a man's dollar, I work to make the man money. The labrats were only interested in their own cut. Understandable - But don't take a banana to a knife-fight if you intend to argue facile syntheses.
I ASSUME these compounds to be more potent - but I can make isophendine for about £400/Kg - see also 'very facile indeed'.
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 511
This agent is worth taking a look at:
BRN 2382808
11-Dimethylamino-9,10-dihydro-9,10-ethanoanthracene
[4053-11-6]

DE1291743 idem Herbert Schroter & Daniel A Prins, US3519718 (1970 to Novartis Corp).
Boissier JR, Ratouis R, Dumont C, Taliani L, Forest J. Synthesis and pharmacological properties of new 9,10-dihydro-9,10-ethanoanthracene derivatives. J Med Chem. 1967 Jan;10(1):86-91. doi: 10.1021/jm00313a018. PMID: 4382229.
Herbert Schroter & Daniel A Prins, US3422104 (1969 to Novartis Corp).

BRN 2382808
11-Dimethylamino-9,10-dihydro-9,10-ethanoanthracene
[4053-11-6]
DE1291743 idem Herbert Schroter & Daniel A Prins, US3519718 (1970 to Novartis Corp).
Boissier JR, Ratouis R, Dumont C, Taliani L, Forest J. Synthesis and pharmacological properties of new 9,10-dihydro-9,10-ethanoanthracene derivatives. J Med Chem. 1967 Jan;10(1):86-91. doi: 10.1021/jm00313a018. PMID: 4382229.
Herbert Schroter & Daniel A Prins, US3422104 (1969 to Novartis Corp).

Last edited:
I think the opioids are likely that way because the natural ligands are polypeptides rather than small molecule transmittersMate - I don't touch stimulants beyond caffeine. I'm quite capable of being anxious without chemicals. In fact, I don't consume anything not prescribed and I don't even like HAVING to take those.
I also find the chemistry sort of dull. Once you have seen a few clandestine labs, you quickly decide that no, you don't want to put ANYTHING made that way into your body.
I like opioid chemistry BECAUSE nobody has been able to find a set of rules. It's a genuine unknown where an independant researcher can genuinely improve upon that which is known. But I don't go anywhere NEAR breaking the law, ever.
Likely other receptors that respond to endogenous polypeptides are similar
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 511
Here's another good one (also made from anthracene):

[1] Rajagopalan, P. (August 1984). "Synthetic CNS agents. 1. (.+-.)-1,2,3,4,4a,5,10,10a-Octahydro-5,10[1',2']-benzenobenz[g]isoquinoline hydrochloride. A new, highly potent, potential antidepressant". Journal of Medicinal Chemistry. 27 (8): 946–947. doi:10.1021/jm00374a002.
[2] Rajagopalan, P. (22 January 1985). "ChemInform Abstract: SYNTHETIC CNS AGENTS. 1. (.+-.)-1,2,3,4,4A,5,10,10A-OCTAHYDRO-5,10(1′,2′)-BENZENOBENZ(G)ISOQUINOLINE HYDROCHLORIDE. A NEW, HIGHLY POTENT, POTENTIAL ANTIDEPRESSANT". Chemischer Informationsdienst. 16 (3). doi:10.1002/chin.198503229.
[3] Parthasarathi Rajagopalan, US4351836 (1982 to Bristol Myers Squibb Pharma Co).
[4] Zhang Hui, CN101696187 (2011 to Xuzhou College of Industrial Technology).

[1] Rajagopalan, P. (August 1984). "Synthetic CNS agents. 1. (.+-.)-1,2,3,4,4a,5,10,10a-Octahydro-5,10[1',2']-benzenobenz[g]isoquinoline hydrochloride. A new, highly potent, potential antidepressant". Journal of Medicinal Chemistry. 27 (8): 946–947. doi:10.1021/jm00374a002.
[2] Rajagopalan, P. (22 January 1985). "ChemInform Abstract: SYNTHETIC CNS AGENTS. 1. (.+-.)-1,2,3,4,4A,5,10,10A-OCTAHYDRO-5,10(1′,2′)-BENZENOBENZ(G)ISOQUINOLINE HYDROCHLORIDE. A NEW, HIGHLY POTENT, POTENTIAL ANTIDEPRESSANT". Chemischer Informationsdienst. 16 (3). doi:10.1002/chin.198503229.
[3] Parthasarathi Rajagopalan, US4351836 (1982 to Bristol Myers Squibb Pharma Co).
[4] Zhang Hui, CN101696187 (2011 to Xuzhou College of Industrial Technology).