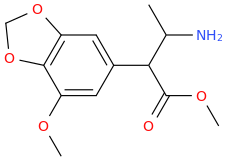
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
🌟🌟 Social 🌟🌟 Rectify's molecular poetry thread
- Thread starter Dresden
- Start date
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 509
Cocabatidine (aka isohomoepibatidine)

Zhang, Chunming; Gyermek, Laszlo; Trudell, Mark L. (1997). "Synthesis of optically pure epibatidine analogs: (1R, 2R, 5S)-2β-(2-chloro-5-pyridinyl)-8-azabicyclo[3.2.1]octane and (1R, 2S, 5S)-2α-(2-chloro-5-pyridinyl)-8-azabicyclo[3.2.1]octane from (−)-cocaine". Tetrahedron Letters. 38 (32): 5619–5622. doi:10.1016/S0040-4039(97)01276-8.
Kim, Yong -Hyun; Won, Do -Youn; Oh, Chang -Young; Woo, Nam -Tae; Park, Young -Ho; Jeong, Jin -Hyun; Ham, Won -Hun (1999). "Stereoselective synthesis of (±)-epibatidine analog: (±)-2β-(2-chloro-5-pyridinyl)-8-azabicyclo[3.2.1]octane". Archives of Pharmacal Research. 22 (3): 300–301. doi:10.1007/BF02976366.
Cocaboxidine (aka isohomoepiboxidine)

Cheng, Jie; Izenwasser, Sari; Zhang, Chunming; Zhang, Suhong; Wade, Dean; Trudell, Mark L. (2004). "Synthesis and nicotinic acetylcholine receptor binding affinities of 2- and 3-isoxazolyl-8-azabicyclo[3.2.1]octanes". Bioorganic & Medicinal Chemistry Letters. 14 (7): 1775–1778. doi:10.1016/j.bmcl.2004.01.025.
UB-165 and Anatoxin A are also agents that are made from natural cocaine.

Zhang, Chunming; Gyermek, Laszlo; Trudell, Mark L. (1997). "Synthesis of optically pure epibatidine analogs: (1R, 2R, 5S)-2β-(2-chloro-5-pyridinyl)-8-azabicyclo[3.2.1]octane and (1R, 2S, 5S)-2α-(2-chloro-5-pyridinyl)-8-azabicyclo[3.2.1]octane from (−)-cocaine". Tetrahedron Letters. 38 (32): 5619–5622. doi:10.1016/S0040-4039(97)01276-8.
Kim, Yong -Hyun; Won, Do -Youn; Oh, Chang -Young; Woo, Nam -Tae; Park, Young -Ho; Jeong, Jin -Hyun; Ham, Won -Hun (1999). "Stereoselective synthesis of (±)-epibatidine analog: (±)-2β-(2-chloro-5-pyridinyl)-8-azabicyclo[3.2.1]octane". Archives of Pharmacal Research. 22 (3): 300–301. doi:10.1007/BF02976366.
Cocaboxidine (aka isohomoepiboxidine)

Cheng, Jie; Izenwasser, Sari; Zhang, Chunming; Zhang, Suhong; Wade, Dean; Trudell, Mark L. (2004). "Synthesis and nicotinic acetylcholine receptor binding affinities of 2- and 3-isoxazolyl-8-azabicyclo[3.2.1]octanes". Bioorganic & Medicinal Chemistry Letters. 14 (7): 1775–1778. doi:10.1016/j.bmcl.2004.01.025.
UB-165 and Anatoxin A are also agents that are made from natural cocaine.
Last edited:
4DQSAR
Bluelighter
- Joined
- Feb 3, 2025
- Messages
- 1,360
Long ago someone asked me to find a possible nicotine alternative and so knowin epibatidine acts on similar sites, I took a deep dive into the various derivatives. At the time several were being investigated as possible new analgesics. The problem researchers always ran into was the toxicity of such compounds.
I don;t think I found the latter compound and it's important because it doesn't contain the 2-chloro-5-pyridyl moiety that appeared to be the cause of toxicity - I suspect because it engendered too much affinity for unwanted subtypes.
But it's 20+ years later and it seems like research in the field has slowed down.
My GUESS is that those biased opiate ligands proved to be more fruitful. I'm ALWAYS very cautious when a startup sells a novel opioid on the basis that it has little to no abuse potential. From buprenorphine to pentazocine to tramasol & tapentadol. Every generation SOMEONE introduces a new class and thusfar, without exception, people abuse them. In fact, sometimes the WAY they are abused actually increases the risk to the user. Mixing first-generation antihistamines seems to reliably increase the subjective euphoria of all opioids.
Tramadol, well, we all saw the number of fatalities that caused and The Lancet reported that in some patients at least, tapentadol produced a particularly nasty dependence, possibly because although it has low affinity, it appears to be a SUPERagonist i.e. it's agonist activity is greater than DAMGO.
I've mentioned it before, but simply replacing the benzylic ethyl side-chain of tapentadol with an N-propyl will produce a much more potent agent. The researchers were VERY careful not to even test anything BUT the ethyl. Clearly they noted that tapentadol overlaid the active conformation of picenadol (whose researcher DID try various chain-lengths). So, given the synthetic simplicity, tahexadol seems a likely future RC. How to get it made? Find a Chinese tapentadol producer - tahexadol would be legal in China and the Chinese are so refreshingly pragmatic when it comes to money. As long as you have a believable story - they WILL believe you. How GOOD the story is will be reflected in the price l-)
I don;t think I found the latter compound and it's important because it doesn't contain the 2-chloro-5-pyridyl moiety that appeared to be the cause of toxicity - I suspect because it engendered too much affinity for unwanted subtypes.
But it's 20+ years later and it seems like research in the field has slowed down.
My GUESS is that those biased opiate ligands proved to be more fruitful. I'm ALWAYS very cautious when a startup sells a novel opioid on the basis that it has little to no abuse potential. From buprenorphine to pentazocine to tramasol & tapentadol. Every generation SOMEONE introduces a new class and thusfar, without exception, people abuse them. In fact, sometimes the WAY they are abused actually increases the risk to the user. Mixing first-generation antihistamines seems to reliably increase the subjective euphoria of all opioids.
Tramadol, well, we all saw the number of fatalities that caused and The Lancet reported that in some patients at least, tapentadol produced a particularly nasty dependence, possibly because although it has low affinity, it appears to be a SUPERagonist i.e. it's agonist activity is greater than DAMGO.
I've mentioned it before, but simply replacing the benzylic ethyl side-chain of tapentadol with an N-propyl will produce a much more potent agent. The researchers were VERY careful not to even test anything BUT the ethyl. Clearly they noted that tapentadol overlaid the active conformation of picenadol (whose researcher DID try various chain-lengths). So, given the synthetic simplicity, tahexadol seems a likely future RC. How to get it made? Find a Chinese tapentadol producer - tahexadol would be legal in China and the Chinese are so refreshingly pragmatic when it comes to money. As long as you have a believable story - they WILL believe you. How GOOD the story is will be reflected in the price l-)
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 509
Well Flubatine is being used on human test subjects. {Based on the name of flubatine I was able to decipher homoepibatidines alternative name Clobatine.}Long ago someone asked me to find a possible nicotine alternative and so knowin epibatidine acts on similar sites, I took a deep dive into the various derivatives. At the time several were being investigated as possible new analgesics. The problem researchers always ran into was the toxicity of such compounds.
I don;t think I found the latter compound and it's important because it doesn't contain the 2-chloro-5-pyridyl moiety that appeared to be the cause of toxicity - I suspect because it engendered too much affinity for unwanted subtypes.
But it's 20+ years later and it seems like research in the field has slowed down.
My GUESS is that those biased opiate ligands proved to be more fruitful. I'm ALWAYS very cautious when a startup sells a novel opioid on the basis that it has little to no abuse potential. From buprenorphine to pentazocine to tramasol & tapentadol. Every generation SOMEONE introduces a new class and thusfar, without exception, people abuse them. In fact, sometimes the WAY they are abused actually increases the risk to the user. Mixing first-generation antihistamines seems to reliably increase the subjective euphoria of all opioids.
Tramadol, well, we all saw the number of fatalities that caused and The Lancet reported that in some patients at least, tapentadol produced a particularly nasty dependence, possibly because although it has low affinity, it appears to be a SUPERagonist i.e. it's agonist activity is greater than DAMGO.
I've mentioned it before, but simply replacing the benzylic ethyl side-chain of tapentadol with an N-propyl will produce a much more potent agent. The researchers were VERY careful not to even test anything BUT the ethyl. Clearly they noted that tapentadol overlaid the active conformation of picenadol (whose researcher DID try various chain-lengths). So, given the synthetic simplicity, tahexadol seems a likely future RC. How to get it made? Find a Chinese tapentadol producer - tahexadol would be legal in China and the Chinese are so refreshingly pragmatic when it comes to money. As long as you have a believable story - they WILL believe you. How GOOD the story is will be reflected in the price l-)

This is much more recent that the older drug tebanicline. I think the logical continuation to that appears to be in a compound called Ropanicant:

I have a lot of agents in my files but a lot of these are more private and not available to view on wikipedia. For example I found an agent that targets the alpha7 receptor called AQW051. Neurosearch did a lot of work in this area and I have lots of their agents in my files. For example, I found an agent called NS3956. I could write a big list but this would be repetitive since I already have all the agents in my list of files. This database link is only a few months old and i've only made a few tweaks in that time:
Last edited:
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 509
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 509
If you want natural alternatives to nicotine, there is lobeline, Cytisine, and arecoline to try.
However, although I am enthusiastic about the work in this area, in terms of what i might actually try, i might look into varenicline.
I tried bupropion once but considered it to have no value. It's interesting to consider if it is permanently a bad drug or just my opinion at the time.
However, although I am enthusiastic about the work in this area, in terms of what i might actually try, i might look into varenicline.
I tried bupropion once but considered it to have no value. It's interesting to consider if it is permanently a bad drug or just my opinion at the time.
4DQSAR
Bluelighter
- Joined
- Feb 3, 2025
- Messages
- 1,360
A did look into all three. The first two were too toxic and too long-acting, The third WAS briefly offered as an RC. Nobody bought it as it turned out that at higher doses (and I know you will be surprised), it turned out to be toxic.
I couldn't quite work out WHY someone would want an alternative. In bulk nicotine is very cheap. Yep, nicotine is also toxic, but it's a known quantity. Making vapes with the others... dodgy.
I couldn't quite work out WHY someone would want an alternative. In bulk nicotine is very cheap. Yep, nicotine is also toxic, but it's a known quantity. Making vapes with the others... dodgy.
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 509
I just forgot to add that Rivanicline might be a suitable alternative to treat nicotine dependency.
Balwinder Singh Bhatti of Targacept Inc is a good chemist to follow.
For example, his name is on the patent of the following inventions: TC-2216, TC-1698, AZD-1446, Epiboxidine, RJR-2429, etc.
Balwinder Singh Bhatti of Targacept Inc is a good chemist to follow.
For example, his name is on the patent of the following inventions: TC-2216, TC-1698, AZD-1446, Epiboxidine, RJR-2429, etc.
4DQSAR
Bluelighter
- Joined
- Feb 3, 2025
- Messages
- 1,360
Well, those chlorinated pyridyl systems always seem to end up too toxic. The thing about nicotine is that it's synthetically simple and although toxic, it's well understood.
Put a novel agent into a vape and if 1 person is harmed - you in trouble.
Put a novel agent into a vape and if 1 person is harmed - you in trouble.
Smyth2
Bluelighter
- Joined
- Jul 26, 2011
- Messages
- 509
The dechloro compound is in the database and is called 3-Pyridin-3-yloxy-1-azabicyclo[2.2.2]octane.

 pubchem.ncbi.nlm.nih.gov
However I cannot find any link to a patent or article on the pubchem website.
pubchem.ncbi.nlm.nih.gov
However I cannot find any link to a patent or article on the pubchem website.
Although I said it is expected to be nicotinic agonist, I would not say to vape it.
For someone who wants an alternative to nicotine to vape with i would suggest developing cytisine or lobeline extracts from plant.
I'm checking my files now and found one called JWB1-84-1 [491878-69-4] & one called JAY2-22-33 [121489-14-3].
[1] Buccafusco JJ, Beach JW, Terry AV. Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328(2):364-370.
[2] Keowkase R, Aboukhatwa M, Adam BL, Beach JW, Terry AV Jr, Buccafussco JJ, Luo Y. Neuroprotective effects and mechanism of cognitive-enhancing choline analogs JWB 1-84-1 and JAY 2-22-33 in neuronal culture and Caenorhabditis elegans. Mol Neurodegener. 2010 Dec 16;5:59. doi: 10.1186/1750-1326-5-59. PMID: 21162742; PMCID: PMC3017027.
[3] Jerry J Buccafusco, et al. WO2003008559 (University of Georgia Research Foundation Inc UGARF, Augusta University Research Institute Inc).
[4] , EP3305785 ().
Jerry Buccafusco was an expert in the field.
3-Pyridin-3-yloxy-1-azabicyclo[2.2.2]octane
3-Pyridin-3-yloxy-1-azabicyclo[2.2.2]octane | C12H16N2O | CID 10058740 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Although I said it is expected to be nicotinic agonist, I would not say to vape it.
For someone who wants an alternative to nicotine to vape with i would suggest developing cytisine or lobeline extracts from plant.
I'm checking my files now and found one called JWB1-84-1 [491878-69-4] & one called JAY2-22-33 [121489-14-3].
[1] Buccafusco JJ, Beach JW, Terry AV. Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328(2):364-370.
[2] Keowkase R, Aboukhatwa M, Adam BL, Beach JW, Terry AV Jr, Buccafussco JJ, Luo Y. Neuroprotective effects and mechanism of cognitive-enhancing choline analogs JWB 1-84-1 and JAY 2-22-33 in neuronal culture and Caenorhabditis elegans. Mol Neurodegener. 2010 Dec 16;5:59. doi: 10.1186/1750-1326-5-59. PMID: 21162742; PMCID: PMC3017027.
[3] Jerry J Buccafusco, et al. WO2003008559 (University of Georgia Research Foundation Inc UGARF, Augusta University Research Institute Inc).
[4] , EP3305785 ().
Jerry Buccafusco was an expert in the field.
Last edited: