MedicinalUser247
Music Ambassador
- Joined
- Aug 2, 2023
- Messages
- 4,747
What about a Psychedelic Opioid ?
N&PD Moderators: Skorpio | someguyontheinternet
What about a Psychedelic Opioid ?
I found a related opioid for sale recently called Spirochlorphine.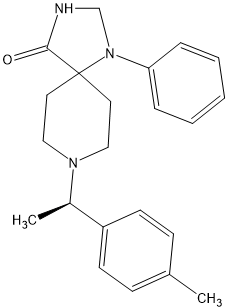
I found the active isomer and discovered that the other isomer had no opioid affinity and so the above is x900 morphine in potency and can be made in 3 steps as the 1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one (which seems like a rare precursor) is in fact used as a precursor to several medicines including Imap, the long-acting neuroleptic often given as a depot medication. Since only 3 manufacturers exists ensure that it is commercially avsailable.
But stronger analogues are known:
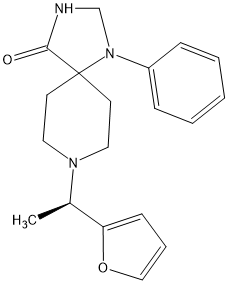
The above is x1100 morphine.
Oh, and in both cases the duration is 8 hours. So 0.25mg TID would prevent a rattle.
SpirochlorphineThis one is called R 6890. [3222-88-6]

Stahl, Kenneth D., Willem Van Bever, Paul Janssen, and Eric J. Simon. “Receptor Affinity and Pharmacological Potency of a Series of Narcotic Analgesic, Anti-Diarrheal and Neuroleptic Drugs.” European Journal of Pharmacology 46, no. 3 (December 1977): 199–205. https://doi.org/10.1016/0014-2999(77)90334-X.
Ketogan is sold as a 1:5 mixture of ketobemidone and Spasmolytic A29.Ketobemidone is one that i would really like to try

Ketogan is sold as a 1:5 mixture of ketobemidone and Spasmolytic A29.

Ketobemidone plus (RS)-3-dimethylamino-1,1-diphenylbut-1-ene (A29) is more potent at NMDA receptors than ketobemidone alone: evidence for A29 as a non-competitive NMDA receptor antagonist - PubMed
The opioid, ketobemidone, has previously been shown to be a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist. In Denmark, ketobemidone is available in a formulation which contains ketobemidone and a spasmolytic compound, (RS)-3-dimethylamino-1,1-diphenylbut-1-ene, hydrochloride...pubmed.ncbi.nlm.nih.gov
me too! I've heard its a little dysphoric but also heard its great fun. I'd love to try it either way, super interesting.Ketobemidone is one that i would love to try. It looks interesting
me too! I've heard its a little dysphoric but also heard its great fun. I'd love to try it either way, super interesting.
Same, but people insist on K-opioid agonists causing dysphoria. Ive heard keto is super great though, it's been on my list of stuff to try for years but never have gotten anywhere close to trying it.I heard it was pretty euphoric more euphoric then heroin for some people
Same, but people insist on K-opioid agonists causing dysphoria. Ive heard keto is super great though, it's been on my list of stuff to try for years but never have gotten anywhere close to trying it.
One can dream...
Here's the synthesis: https://ibb.co/w7fm10wUS3238216A
There is quite a lot out there but while I find it interesting, it's clear that unless one has access to 1-Phenyl-1,3,8-triazaspiro[4.5]decan-4-one (CAS 1021-25-6). it's a LOT of work.
There are more facile targets. Hence brorphine, I assume.