Image n hosted in ImgBB

ibb.co
From 'Opiates' by Lenz et al.,
Page 441, Compound 82.
A. Wilson and A. W. Pircio, Nature
volume 206, pages1151–1152 (1965) (London) 206, 1151 (1965)
'Narcotic Analgesics: Possibility of Broadening the Structural Basis of Analgesics'
See also:
Journal of the American Chemical Society Volume 86/Issue 22
'Ring Openings of Substituted Cyclobutanes Induced by Grignard Reagents. I. Methyl 2-Dimethylamino-3,3-dimethylcyclobutanecarboxylate'
Leonard. Weintraub Armin. Wilson David L. Goldhamer Donald P. Hollis
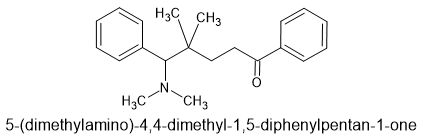
Anyone else notice that the very first novel opioid in this thread (image provided) is surprisingly similar to the paper and patent I referenced in my previous post?
It does suggest that μ activity (agonist - Example 4a) may be enhanced with a benzylic ketone or, if this part of the structure binds in a similar manner to fentanyl, an (S) beta hydroxy moiety.
Sadly I have been unable to find any information of the activity of the two isomers. I know from personal communication with Dan Lednicer (now RIP) that once BDPC had been discovered, the team was so surprised at it's potency in animal models that they felt there was no benefit in further research. Even MDPC wasn't tested. I asked why and his answer was simple 'In all the excitement, we forgot'. This is the stuff you don't read in journals, folks!
In retrospect, the p-Me is a much better target in many ways. It provides a sacrificial moiety i.e. that p-Me is very likely to be the major metabolic pathway via oxidation. The few reports on BDPC appear to suggest that it's fine for the first four hours or so, then it becomes deeply unpleasant. My best guess on this is that N-demethylation is the major metabolic pathway and the monomethyl metabolite has significant kappa activity.
A Chinese team confirmed that the beta aromatic ring of BDPC could be swapped and the increased activity of C-8813 confirmed whatever their research was designed to show. As far as I know, nobody tried substituting the cyclohexanol ring or adding a beta ketone/hydroxyl moiety. Or, if they did, I cannot find and references to that work.

 pubchem.ncbi.nlm.nih.gov
[413587-97-0]
pubchem.ncbi.nlm.nih.gov
[413587-97-0]