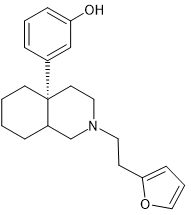
Actually, a THF ring might work thus:
Image 1 hosted in ImgBB

ibb.co
Now the above will be more rigid but one might have to move dihydrofuran and their would have to be enough space in receptor BUT it forces that terminal -CH3 into a specific space. Maybe furan (being slightly smaller) would alter activity but it's never been tried.
WO-2007091950-A1
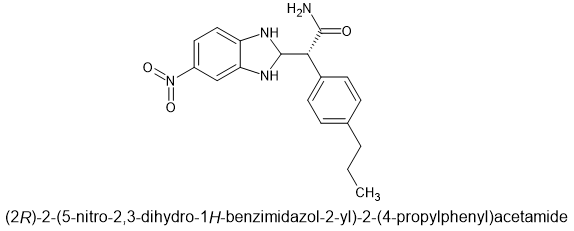
Comounds with the THF are known but they also have CB1 affinity. So maybe the parent has some CB1 affinity? The amide side-chain provides NOP affinity so although it's analgesia is 4 times that of the parent (if chiral), you might get more out of it.
But If the lead compound is x60 and THAT is worth making, the derivative with the carboxamide should be worth it. After all, all one has to do is have a -C≡N by heating in solution of water, tert-butyl alcohol and LiOH (why LiOH is preferred isn't something I bothered to look up. Also there is work using Cu catalysts that are chiral but their are simple ways to resolve and dehydrate unwanted isomer.
I suspect it's not going to cost twice as much... but just as fentanyl is being made by 'cooks', the simple route to this class leads me to suspect that again, it's 'cooks' who earn about £250/day. I'm guessing they are staying ahead of the law.
But as I said, their is a compound legal everywhere but UK that is x3600 M in 3 steps - and that's a figure derived from tests on multiple species so it's a robust figure. Much easer to make 1Kg of this rather than 45Kg of fentanyl. Oh, and it's very euphoric. So if fentanyl wholesales for $4150.... well, work it out... x45.