aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
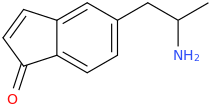
Okay, first off, say in cyclopentane, if the bond lengths were all the same, one could make a reasonable claim that it would adopt a planar conformation because this would give it the shape of a regular pentagon with an angle of 108 degrees between carbons, very close to the 109.5 degrees a normal tetrahedral carbon is in. So one could argue that in the planar conformation it is least strained and so is of lower energy than other conformations which would result in more strain.
But, 2 problems. First, cyclopentane itself is not observed to be planar but adopts a lower energy "envelope" conformation. Now, the reason why this is lower energy is something I'm not sure why this is the case and any insight into this problem would be good, because it seems that with this conformation there would be more overall ring strain.
https://en.wikipedia.org/wiki/Cyclopentane#/media/File:Cyclopentane3D.png
https://en.wikipedia.org/wiki/Cyclopentane#/media/File:Cyclopentane_halfchair.svg
The second problem is that the bond lengths are not the same. In the 5 carbons of indane, the benzene carbons probably have a distance of close to 1.40 angstroms and all other carbons are closer in distance to 1.54 angstroms. This is not a regular pentagon and thus the angles would not be the same if it were to adopt a planar conformation. So there would be more ring strain if it adopted planar. It can reduce this ring strain by depressing itself so that the distance between all the carbons are kept the same but the angles are as unstrained as possible. I'm trying to figure out right now what this angle of depression would be.
Finally however, on the wikipedia page of indane, the molecule does indeed look non-planar and that image is probably from a program which energy minimises the structure first.
https://en.wikipedia.org/wiki/Indane
But, 2 problems. First, cyclopentane itself is not observed to be planar but adopts a lower energy "envelope" conformation. Now, the reason why this is lower energy is something I'm not sure why this is the case and any insight into this problem would be good, because it seems that with this conformation there would be more overall ring strain.
https://en.wikipedia.org/wiki/Cyclopentane#/media/File:Cyclopentane3D.png
https://en.wikipedia.org/wiki/Cyclopentane#/media/File:Cyclopentane_halfchair.svg
The second problem is that the bond lengths are not the same. In the 5 carbons of indane, the benzene carbons probably have a distance of close to 1.40 angstroms and all other carbons are closer in distance to 1.54 angstroms. This is not a regular pentagon and thus the angles would not be the same if it were to adopt a planar conformation. So there would be more ring strain if it adopted planar. It can reduce this ring strain by depressing itself so that the distance between all the carbons are kept the same but the angles are as unstrained as possible. I'm trying to figure out right now what this angle of depression would be.
Finally however, on the wikipedia page of indane, the molecule does indeed look non-planar and that image is probably from a program which energy minimises the structure first.
https://en.wikipedia.org/wiki/Indane