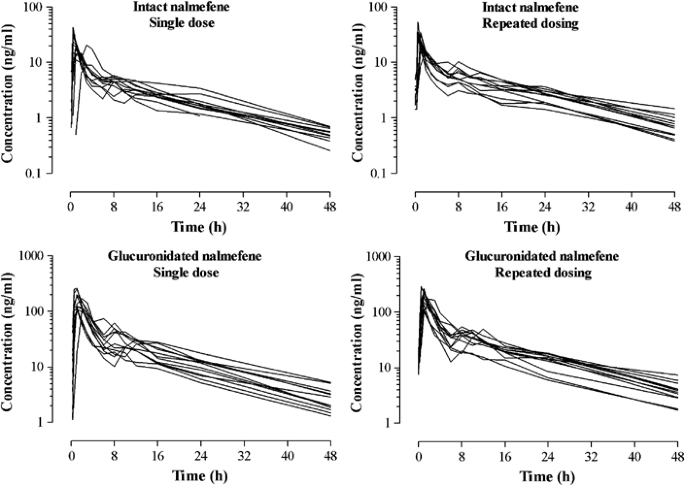
DeathIndustrial88
Bluelighter
- Joined
- Jan 23, 2020
- Messages
- 2,943
My vote is to definitely sleep on it a night or two.I'm actually considering deleting this thread just like I did with the other two science related threads I created, because sometimes I sometimes feel like this forum doesn't deserve such threads. I mean just look at the replies. For god's sake, instead of all coming together (regardless of anyone's level of knowledge, we are ALL lifelong students.) and COLLABORATING together (the real reason I have created these threads) to discuss, discover, ask, answer and perhaps even create something together, it ends up becoming a childish dick-measurement contest where people just try to show everyone else how right they are and just how much they know in more or less subtle patronizing ways. If then someone dares to voice legitimate criticism, various logical fallacies are used, from appeal to authority to the accomplishment fallacy (muh experience, so shut up you greenhorn), and the worst of all: straight up LIES! Putting words and intentions into my mouth that I have NEVER actually said or meant. A forum with such members does NOT deserve such threads. I'm gonna take these things to an actual chemistry and pharmacology forum (there is a good german one I know of, even though it has like only 5 active members, but at least they are humble and open to being corrected despite having a billion years of experience under their belt, which btw doesn't protect you from being wrong) which is a place better suited for such discussions where people actually collaborate together.
Also funny how AT told me the entire time how ER/SR doesn't exist and is merely a "political" designation, but then when Skorpio literally said the same thing that I said (OROS System) he suddenly turned around and was like "oh uh yeah, that OROS thing...pretty cool stuff mate". So the exact same thing you told me happened in that bupe thread and why you eventually stopped replying to such an hopeless case has repeated itself here. These kind of things just show me that there is some personal ego stroking shit going on behind posting such "educational" replies here and not an actual desire TO TEACH AND BE TAUGHT IN LIKE MANNER.
I think I'll sleep one or two nights over this and then decide whether or not I delete this thread. I have copied my entry post and translated it in german in case I do delete it so I can then post it in the german pharmachem forum.
Hey no hurries buddy. Take your time, ok? It ain't like I'm sitting here all day and wondering when doomy-gloomy deathy industrially (hehe I like to cutify other people's names...tehehe) will finally reply to my emails. So chiiiilllllll
EDIT:
earlier I said "A forum with such members does NOT deserve such threads". That was perhaps not really fair, as 99% of all members I have talked to here ever since I registered are completely fine. It wasn't my intention to generalize. It's just that the remaining 1% are very vocal and acive and can make or break a thread. That's what I wanted to say and not that BL as a whole is responsible. You guys are great, especially the staff!
I think there's still some non-confrontational people here who can benefit from the information provided here.
I've actually lurked on BL for the past 10 years but only a made an account within the past few years. So there could be countless people out there gaining insight from things who aren't even active on here as well.
But in all honesty, I'd completely understand why you would. Especially if it keeps going the way it's going. It's kind of ironic that I told you that this is what people do & then it basically happened to you too. People trying to tell me I'm wrong about shit left & right, only to say the exact same thing I said (just slightly different) in another reply. lol I don't get it at all. I have absolutely zero problems with being educated or told what's right or correct as I enjoy learning (to the best of my ability) anyway but that's not what's actually happening here. Just people needing their ego's stroked to the point where they're willing to deflect & even just make shit up. lol
Awe, but I want you to wonder about me!!!
Just left you another novel to read!! I enjoy getting back to you anyway! I was even gonna keep going haha But then you'd have too much stuff to respond to, so I'll chill. lol
Off-topic : I made $50 bucks yesterday selling cassettes I made. But now I have to go to the damn post office & stand in line for 20hrs to send it out. lol Hoping it goes well cause I definitely don't have the patience to deal with the public today.

 Maybe it is & just isn't used much.
Maybe it is & just isn't used much.
 to Hexenstahl. Its a great forum most use drugs and sometimes thread's derail. No need to use spells. Kiddin'
to Hexenstahl. Its a great forum most use drugs and sometimes thread's derail. No need to use spells. Kiddin'