Hexenstahl
Bluelighter
- Joined
- Jan 4, 2022
- Messages
- 1,017
God I'm spending too much time on pubchem again, looking and comparing chemical/physical properties and formulas and structures between various opioids lol. I'm going to dedicate this thread to the humble and incredibly underrated, almost unknown Madame Tilidine, which has an equianalgetic potential of only ~0.1 - 0.2 compared to morphine, but is THE most euphoric opioid I ever had the pleasure of trying while still in my opioid naive phase 12 years ago (back then I tried everything from codein, to tramadol, to DHC from a famous british brand which an english friend of mine smuggled to Germany for me while visiting me, and finally to Tilidine which I loved more than anything else). In fact Tilidine is so notorious here in Germany for its euphoria and lack of sedation, that the german-swiss drug forum "Eve & Rave" has an entire thread dedicated to questions and discussions surrounding that opioid back from 2007 spanning 389 pages to this day! That's how popular it is here!
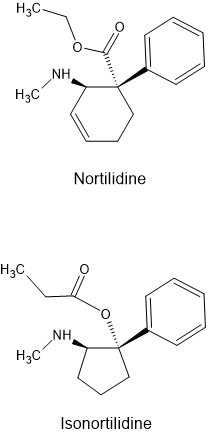
Now, this fully synthetic opioid has quite an interesting skeletal formula which reminds me a bit of Levomethadone, but it's kind of diagonal (this has to be one of the most unscientific descriptions that I have ever come up with lol, but I'm lazy right now, so please cut me some slack).
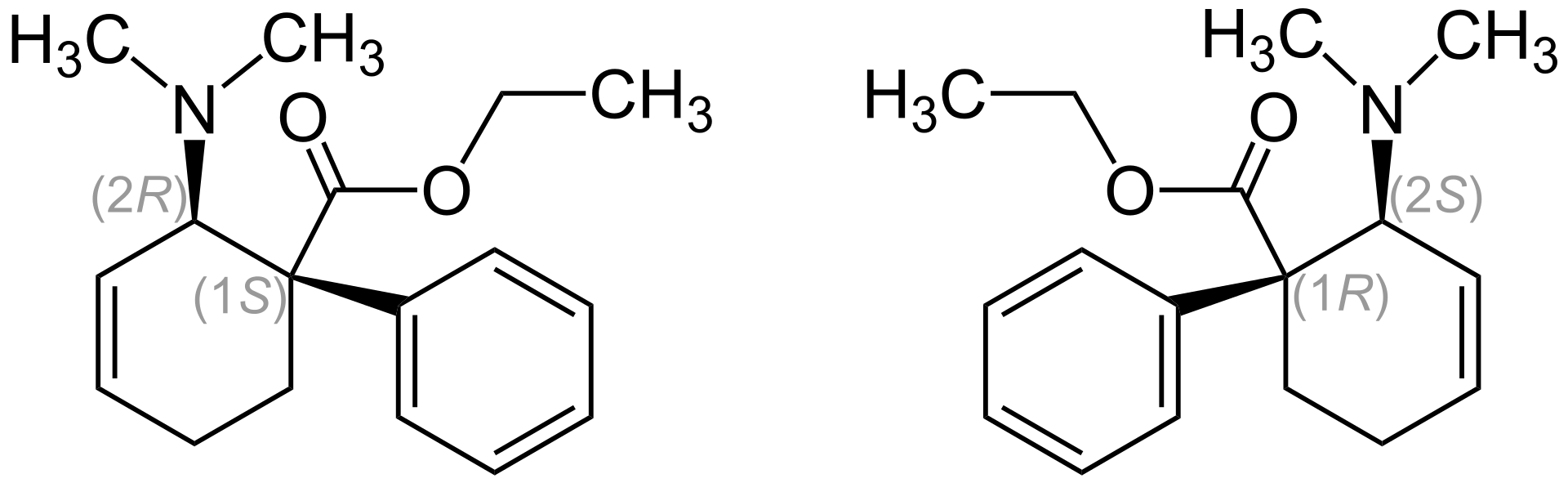
This is the 1:1 stereoisomer mixture of (1S,2R)-Isomer (left) und (1R,2S)-Isomer (right). The 2-(Dimethylamino)-1-phenylcyclohex-3-en-carboxylic acid-ethylester has two stereogenic centers. So there are actually 4 stereoisomers: The enantiomeric (1R,2S) and (1S,2R) form, as well as (1R,2R) and (1S,2S). Tilidine is a racemat which is made out of the enantiomers (1R,2S) and (1S,2R). The importance of enantiomeric purity has become more and more important over time it seems, because the enantiomers of chiral drugs (almost) always show different pharmacological and pharmacokinetic profiles. Just to give you an idea of how the slighest deviations in purity can have an enormous impact: the trans-form has twice the activity than the cis-form (which isn't commercially used for obvious reasons) and that only because the cis-racemat has an impurity of 0.5%!!!
The (1S,2R) enantiomer [(+)-trans] is a whole lot more stronger in terms of activity than the (1R,2S) enantiomer [(−)-trans] (ED50 is 23:1 when s.c. ROA is applied).
Tilidine itself is actually a prodrug. It is only when the liver enzymes remove a methyl group from the molecule, turning tilidine into nortilidine, that turns the drug into a psychoactive opioid. Nortilidine is an incredibly fast acting opioid with effects already setting in within 10 to 15 minutes (oral ROA). When I took the Tilidine oral solution I started to feel the first effects within that time period. Interestingly though, the effect wears off quite slowly.
Papers say that Tilidine has no antitussive effect but I disagree. I have found the antitussive effect to be quite weak, but it's definitely there.
Now, I don't know much about the synthesis of this wonderful opioid, but the precursors are quite interesting. Crotonaldehyde and dimethylamine are turned, with atropic acid ethyl ester, into dimethylaminobuta-1,3-dien via the Diels-Alder-Reaction. I don't know the steps involved to turn the latter compound into Tilidine though.
Let's look at crotonaldehyde and dimethylamine. The former precursor is found in a plant called "Croton". This plant has an oil, the croton oil, which can be turned into crotonaldehyde. What's interesting is that this substance is also found in the human body. Polyamines turn ethanal into crotonaldehyde after alcohol consumption.
This precursor is highly flammable, with a strong, piercing smell, is very hard to solve in water (better solubility can be achieved in organic solvents) and tends to build peroxides and atoxidation in the presence of oxygen.
Dimethylamine is also an interesting precursor, not because of how it is synthesized or used, but due to the fascinating and yet relatively unknown fact that it is found in cannabis smoke. If the %-composition of DMA is big enough, cannabis smoke could be used as another source to produce DMA (if self-grown, otherwise it's too expensive ofc).
All in all, Tilidine is a very interesting opioid, both chemically as well as psychopharmacologically. If I wanted to synthesize a potent opioid with a strong euphoric response, I'd look towards Tilidine as a potential candidate and further manipulate it chemically to increase its analgetic potency. Tilidine as it stands today is simply too weak potency wise, but has a lot of potential if a pharmaceutical chemist had the interest and determination to create a much more potent derivative of this opioid. I think I'll do that in the future

Now, this fully synthetic opioid has quite an interesting skeletal formula which reminds me a bit of Levomethadone, but it's kind of diagonal (this has to be one of the most unscientific descriptions that I have ever come up with lol, but I'm lazy right now, so please cut me some slack).

This is the 1:1 stereoisomer mixture of (1S,2R)-Isomer (left) und (1R,2S)-Isomer (right). The 2-(Dimethylamino)-1-phenylcyclohex-3-en-carboxylic acid-ethylester has two stereogenic centers. So there are actually 4 stereoisomers: The enantiomeric (1R,2S) and (1S,2R) form, as well as (1R,2R) and (1S,2S). Tilidine is a racemat which is made out of the enantiomers (1R,2S) and (1S,2R). The importance of enantiomeric purity has become more and more important over time it seems, because the enantiomers of chiral drugs (almost) always show different pharmacological and pharmacokinetic profiles. Just to give you an idea of how the slighest deviations in purity can have an enormous impact: the trans-form has twice the activity than the cis-form (which isn't commercially used for obvious reasons) and that only because the cis-racemat has an impurity of 0.5%!!!
The (1S,2R) enantiomer [(+)-trans] is a whole lot more stronger in terms of activity than the (1R,2S) enantiomer [(−)-trans] (ED50 is 23:1 when s.c. ROA is applied).
Tilidine itself is actually a prodrug. It is only when the liver enzymes remove a methyl group from the molecule, turning tilidine into nortilidine, that turns the drug into a psychoactive opioid. Nortilidine is an incredibly fast acting opioid with effects already setting in within 10 to 15 minutes (oral ROA). When I took the Tilidine oral solution I started to feel the first effects within that time period. Interestingly though, the effect wears off quite slowly.
Papers say that Tilidine has no antitussive effect but I disagree. I have found the antitussive effect to be quite weak, but it's definitely there.
Now, I don't know much about the synthesis of this wonderful opioid, but the precursors are quite interesting. Crotonaldehyde and dimethylamine are turned, with atropic acid ethyl ester, into dimethylaminobuta-1,3-dien via the Diels-Alder-Reaction. I don't know the steps involved to turn the latter compound into Tilidine though.
Let's look at crotonaldehyde and dimethylamine. The former precursor is found in a plant called "Croton". This plant has an oil, the croton oil, which can be turned into crotonaldehyde. What's interesting is that this substance is also found in the human body. Polyamines turn ethanal into crotonaldehyde after alcohol consumption.
This precursor is highly flammable, with a strong, piercing smell, is very hard to solve in water (better solubility can be achieved in organic solvents) and tends to build peroxides and atoxidation in the presence of oxygen.
Dimethylamine is also an interesting precursor, not because of how it is synthesized or used, but due to the fascinating and yet relatively unknown fact that it is found in cannabis smoke. If the %-composition of DMA is big enough, cannabis smoke could be used as another source to produce DMA (if self-grown, otherwise it's too expensive ofc).
All in all, Tilidine is a very interesting opioid, both chemically as well as psychopharmacologically. If I wanted to synthesize a potent opioid with a strong euphoric response, I'd look towards Tilidine as a potential candidate and further manipulate it chemically to increase its analgetic potency. Tilidine as it stands today is simply too weak potency wise, but has a lot of potential if a pharmaceutical chemist had the interest and determination to create a much more potent derivative of this opioid. I think I'll do that in the future
Last edited: