nirvus
Bluelighter
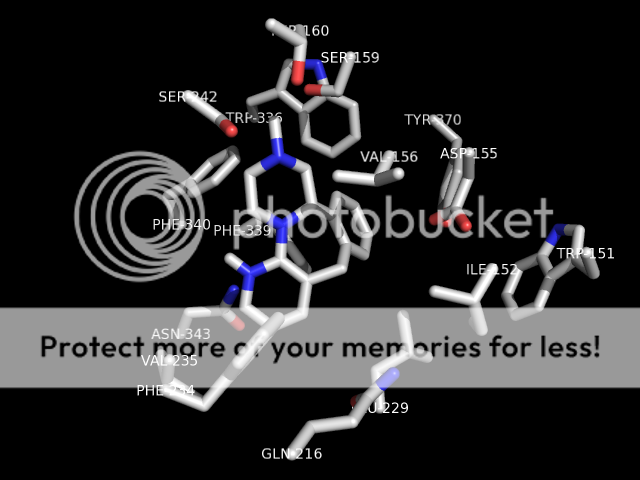
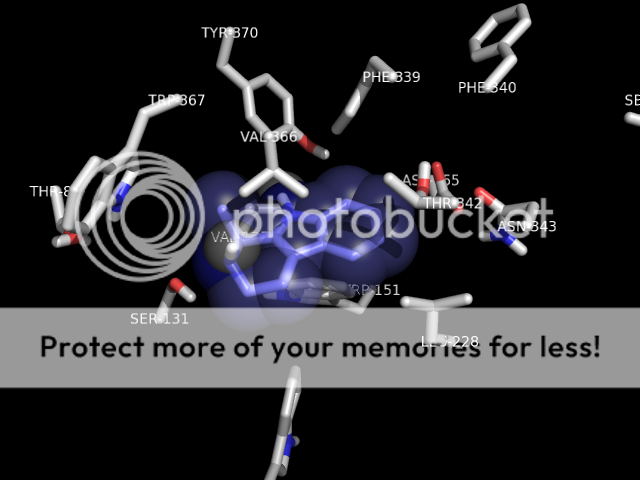
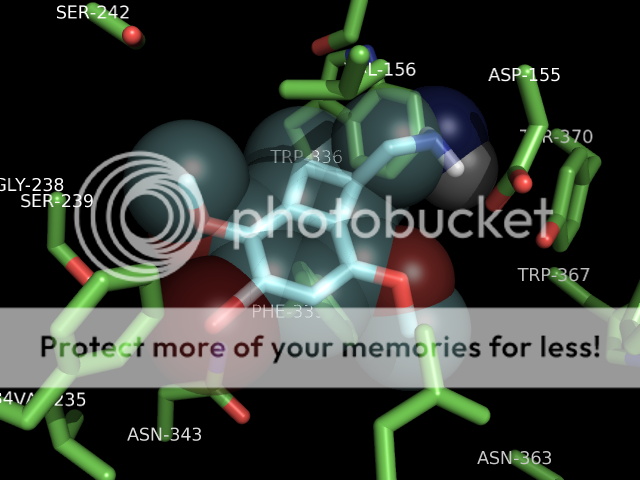
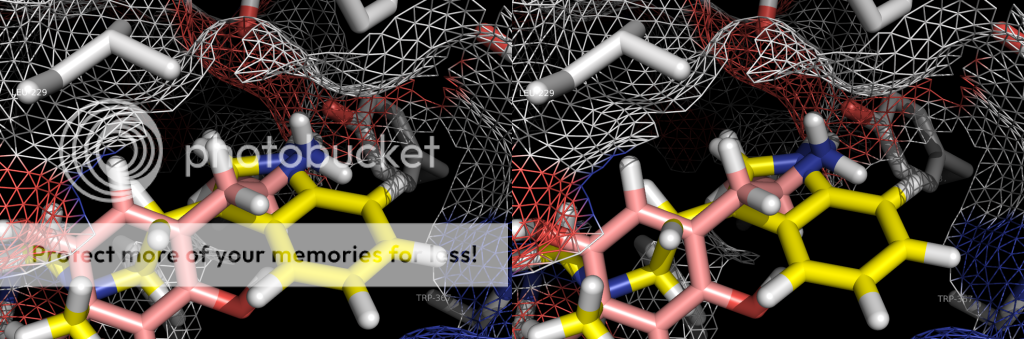
p.s. someone i know personally who was intolerant of SSRI's side effects was briefly prescribed mirtazapine. she does not care much for serotonergics at all (hence intolerant of SSRI therapy) but noted that mirtazapine would quickly KILL a trip and allow sleep. it would cut right through a low-dose (5-10mg) 2c-e experience. i know this has been discussed elsewhere, but just my 2 cents. seems hospital emergency room/EMT's could maybe utilize a med with a pretty good safety profile to combat hallucinogen overdose. i think benzo's are weapon of choice currently ,which ain't bad, but this stuff would go right to the crux of the problem (at the 2a receptor). plus the strong antihistamine activity is fairly sedative. anyway. . .
edit: yay! 50 posts.
edit: yay! 50 posts.
Last edited: