-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Lucigenol
- Thread starter AuraithX
- Start date
Ham-milton
Bluelighter
- Joined
- Jul 20, 2007
- Messages
- 5,746
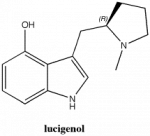
(R)-3-((1-methylpyrrolidin-2-yl)methyl)-1H-indol-4-ol. An absolutely horrible name choice- that's the name of a chemical in glow sticks.
Doesn't look like it's very stable, and according to Vector the 5-MeO analogue was tested in humans and wasn't great.
Doesn't look like it's very stable, and according to Vector the 5-MeO analogue was tested in humans and wasn't great.
fastandbulbous
Bluelight Crew
- Joined
- Jul 29, 2004
- Messages
- 21,304
dbailey11 said:What's it supposed to be, a stimulant or a psych?
It's a psychedelic with a lot of sedative-like properties (not much cop IMO). Active at 1-1.5mg in humans
It's an analog of LSD.
It's not as it doesn't have any C or D ring like LSD. If it has to be an analogue of anything, I'd vote for AMT/5-methoxyAMT by it's structure (alpha methyl extended and attached to amine,with additional methyl group added to give a 5 membered heterocyclic structure)
MurphyClox
Bluelighter
- Joined
- Mar 26, 2008
- Messages
- 1,416
I only know about in vitro trials and in vivo, using rats; binding studies (...conformational energies) suggest a different binding mode than LSD at the 5HT2A-receptor. It's said to have around a 10th of the potency of LSD...
fastandbulbous
Bluelight Crew
- Joined
- Jul 29, 2004
- Messages
- 21,304
It's weird in that the stereochemistry around C5 of the most active isomer is the opposite of the conformation of all the other optically active compounds that are psychedelic 5HT2as agonists.
As regards activity, I'm quoting directly from some communication from a synthetic organic chemist (with a liking for psychedelics!) I know. Personally, the methoxy group in the indolic 5 position is a good enough reason for me to avoid it...
As regards activity, I'm quoting directly from some communication from a synthetic organic chemist (with a liking for psychedelics!) I know. Personally, the methoxy group in the indolic 5 position is a good enough reason for me to avoid it...
Riemann Zeta
Bluelighter
- Joined
- Apr 21, 2004
- Messages
- 1,655
I have heard small whispers of a psychedelic with the name "lucigenol." No one seems to know what the exact structure that corresponds with this name is? For some reason, the claim has been made that it is analogue of LSD, which it is clearly not. Also, some think that it is a 5-MeO compound, others a psilocin-like 4-OH tryptamine (with maybe 10x the potency of psilocin). Can anyone confirm that this compound (first synthesized by Nichols) has actually been officially named "lucigenol" and has actually been tested by humans as a psychedelic? If so, why would it be predicted to have a strong sedative effect?
Note: the (R)-enantiomer is the more active isomer from the paper.
Note: the (R)-enantiomer is the more active isomer from the paper.
Attachments
Jabberwocky
Frumious Bandersnatch
- Joined
- Nov 3, 1999
- Messages
- 84,998
I thought it was sedating since it was an analogue of LSA or some such? I could be totally off base though.
*ss wants a sedating psych*
*ss wants a sedating psych*
Ham-milton
Bluelighter
- Joined
- Jul 20, 2007
- Messages
- 5,746
Are you a member of Blacklight.in samadhi? I've posted a lot of (what I hope is) really good information about the structural activity relationships of depressants.
Most relevant to your particular desires has to be the GABA-A a1-selective ligands. These seem to require a pentagonal ring w/ one or more nitrogens (three seems to be the max, but I'm not sure) and usually one or more double bonds, but thalidomide has none, but I can't find data on it's selectivity. Off of that a chain that looks like this: C-C(=O)-N, with the N usually being disubstituted (except of course, again in thalidomide and CL-218-872).
This basic structure should make the potential psychedelic-sedatives obvious: it's obviously something that the basic tryptamine structure can be modified to accomodate, simply changing the amine to an amide (ie: a ketone on the alpha carbon).
I can't find anything on the 5HT2a selectivity or the sort of potency we could expect from these.
Obviously the hope would be for much stronger preference for 5HT2a over GABA-A a1-subunit containing receptors since a1 agonists are so well known as strong hypnotics and amnesics.
I think that these might make great potential psychedelics for the anxiety-impaired. I'm certainly one of these, so I've been quite restricted from using psychedelics except when I've had a long period of preperation before partaking in any of the sacraments.
With PTSD and potentially unsettling, upsetting and unwanted flashbacks so common after a particularly bad trip (they are of course extremely uncommon on the whole with psychedelic users, but the risk goes greatly up among those unfortunate enough to have had a really bad trip), these offer two advantages: they'd be less prone to inducing bad trips, and if one were to develop, as they certainly can (look at the bad 'trips' resulting from Zolpidem), taking a higher dose could be used to produce complete amnesia of the experience.
A lot of the more new-agish psychedelic users will be quick to say "that won't solve anything, that'll simply push it into the back of the mind and repress the experience." However, when considering the horrible things that have happened to and done by people under the influence of GABAergic drugs that produced complete amnesia, and don't seem to harm or even affect the experiencee unless discovered through some other route, I think it's safe to say that it's simply hippy hogwash to borrow a phrase I once heard at a George Bush 2004 rally.
(to think, as I originally wrote all that, it was only one sentence, and now it's only two! I could never parody Kurt Vonnegut, that's for sure)
Most relevant to your particular desires has to be the GABA-A a1-selective ligands. These seem to require a pentagonal ring w/ one or more nitrogens (three seems to be the max, but I'm not sure) and usually one or more double bonds, but thalidomide has none, but I can't find data on it's selectivity. Off of that a chain that looks like this: C-C(=O)-N, with the N usually being disubstituted (except of course, again in thalidomide and CL-218-872).
This basic structure should make the potential psychedelic-sedatives obvious: it's obviously something that the basic tryptamine structure can be modified to accomodate, simply changing the amine to an amide (ie: a ketone on the alpha carbon).
I can't find anything on the 5HT2a selectivity or the sort of potency we could expect from these.
Obviously the hope would be for much stronger preference for 5HT2a over GABA-A a1-subunit containing receptors since a1 agonists are so well known as strong hypnotics and amnesics.
I think that these might make great potential psychedelics for the anxiety-impaired. I'm certainly one of these, so I've been quite restricted from using psychedelics except when I've had a long period of preperation before partaking in any of the sacraments.
With PTSD and potentially unsettling, upsetting and unwanted flashbacks so common after a particularly bad trip (they are of course extremely uncommon on the whole with psychedelic users, but the risk goes greatly up among those unfortunate enough to have had a really bad trip), these offer two advantages: they'd be less prone to inducing bad trips, and if one were to develop, as they certainly can (look at the bad 'trips' resulting from Zolpidem), taking a higher dose could be used to produce complete amnesia of the experience.
A lot of the more new-agish psychedelic users will be quick to say "that won't solve anything, that'll simply push it into the back of the mind and repress the experience." However, when considering the horrible things that have happened to and done by people under the influence of GABAergic drugs that produced complete amnesia, and don't seem to harm or even affect the experiencee unless discovered through some other route, I think it's safe to say that it's simply hippy hogwash to borrow a phrase I once heard at a George Bush 2004 rally.
(to think, as I originally wrote all that, it was only one sentence, and now it's only two! I could never parody Kurt Vonnegut, that's for sure)
Ham-milton
Bluelighter
- Joined
- Jul 20, 2007
- Messages
- 5,746
wow, that is potent
Riemann Zeta
Bluelighter
- Joined
- Apr 21, 2004
- Messages
- 1,655
I personally like a nice stimulating psychedelic (the more amphetaminergic the better, as long the PNS side effects aren't too annoying), so I guess this one is not for me. Then again, I guess I've been lucky in the fact that I've never had a bad trip.
fastandbulbous
Bluelight Crew
- Joined
- Jul 29, 2004
- Messages
- 21,304
MattPsy said:(R) isomer is a lot more potent, I forget how much. Look at the paper if u want to know.
Binding affinity for 5-HT2A receptor is 13 +/- 2 nM (Ki [125I]DOI).
Is that the 4-hydroxy compound? I was talking about the 5-methoxy one.
Crossed wires...
AuraithX
Bluelighter
- Joined
- May 22, 2006
- Messages
- 4,504
I have 2 tabs of this stuff.
Does anyone have anymore info for me? Seems there are no trip reports anywhere :/
If someone is making it someone must've taken it by now!
edit: ah! found one..
http://www.drugs-forum.co.uk/forum/showthread.php?p=411358
Does anyone have anymore info for me? Seems there are no trip reports anywhere :/
If someone is making it someone must've taken it by now!
edit: ah! found one..
http://www.drugs-forum.co.uk/forum/showthread.php?p=411358