-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
@navarone, I don't believe ethylene glycol toxicity would be much of an issue at recreational or therapeutic doses of fenethylline. Assuming complete hydrolysis, 100 mg fenethylline would yield about 18 mg ethylene glycol and 40 mg amphetamine. That's nowhere near a toxic dose of ethylene glycol. Increasing the dose, you'd OD on amphetamine long before EG toxicity would get at you. Fenethylline is only partly metabolised, and if we trust the Wiki entry claiming that 25.4% ends up as amphetamine and 13.7% as theophylline, the actual figure on EG would be even smaller (you'd probably get some hydroxyethyltheophylline and hydroxyethylamph as well).
Water with less than 7 mg EG per litre is considered safe for drinking. It's probably not healthy, but neither are amphetamines.
Water with less than 7 mg EG per litre is considered safe for drinking. It's probably not healthy, but neither are amphetamines.
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Ethylene glycol is a natural constituent of e.g. fruit juices and fermented drinks. Just like methanol, it's fine to have traces of it in your food/drink (thats why oxidative metabolism exists) as long as you don't overload your body's pathways for handling the metabolites...
- Joined
- Jul 21, 2002
- Messages
- 12,042
Here we go:
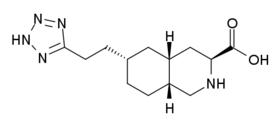
Good stuff, definitely something I haven't heard off.......
But, it would be interesting to have a variant along the lines of this existing neurosteroid.........
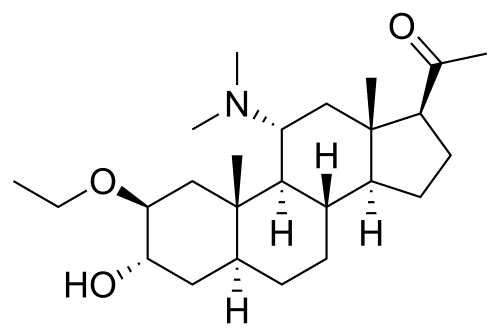
Yet.......tone, down the GABAergic properties, add some mild pro-anabolic/androgenic qualities......and perhaps, an indirect opioidergic or dare I say, modestly potent opioidergic metabolite (we can dream).........
Or just piece together a hideous beast that is pro-drug for multiple conventional compounds with such properties (requiring a "routine" periodic hepatic transplantation).......
you coulda left the original methylenedioxys too
Now THAT would be pushing it.
/navarone/
Bluelighter
- Joined
- Dec 26, 2003
- Messages
- 1,322
Did anyone else here get really exited before trying 6-APB (Benzofury) but was later very dissapointed apart from being fucked up by the harsh comedown?
The molecule looked pretty promising considering the aromaticity/electronegativity around the 4th and 5th position and the avoidance of having a-methyldopamine as a metabolite. It sucked pretty much and the psychotic like comedown was horrible.
I first thought 5-APB would have been better thinking that the oxygen at the 4th position would mimic MDMA even more though I later figured out that it was a less desired substance hence its popularity being below that of benzofury.
I yet have to find or try a decent MDMA substitute with fewer risks of toxicity, an analogue with more reuptake inhibition rather than releasing properties was my first thought but in my honest though limited experience with DNRIs and TRIs, reuptake inhibitors are pretty dissapointing compared to amphetamines reason why I find coke almost worthless as a stimulant.
The molecule looked pretty promising considering the aromaticity/electronegativity around the 4th and 5th position and the avoidance of having a-methyldopamine as a metabolite. It sucked pretty much and the psychotic like comedown was horrible.
I first thought 5-APB would have been better thinking that the oxygen at the 4th position would mimic MDMA even more though I later figured out that it was a less desired substance hence its popularity being below that of benzofury.
I yet have to find or try a decent MDMA substitute with fewer risks of toxicity, an analogue with more reuptake inhibition rather than releasing properties was my first thought but in my honest though limited experience with DNRIs and TRIs, reuptake inhibitors are pretty dissapointing compared to amphetamines reason why I find coke almost worthless as a stimulant.
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
I revamped the "List of Cocaine Analogues" page at Wikipedia quite a bit. It's still far from perfect (or even to holding candles to the Phenyltropane list page)
...and in commemoration of that bit of mental masturbation:
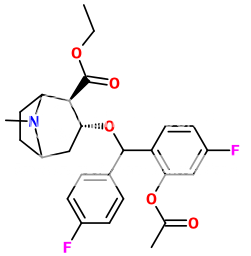
2'-acetoxy-difluro-ethyl-pine
I've always thought cocaine looked too dualistic, with the two rings & two acetyl branches. 2'-acetoxy-cocaine remedies the branches to a nice triad, but the core of two rings are maintained. Difluoropine is the answer for the rings. I turned the methyl into an ethyl for good measure. However, since due to the difluoropine conformation, I had to reverse the stereochemistry for the optimal isomer, with the 2'-acetoxy branch, it's anybodies guess how it may function. (For instance, it may requiring being on the ring with the other fluorine fluorocarbon, which wouldn't alter my aesthetic specifications)
(edit: it looks a bit like those "BromoDragonFly" molecules, doesn't it? With the tropane as the tail, and the 2'-acetoxy as the head.)
...and in commemoration of that bit of mental masturbation:

2'-acetoxy-difluro-ethyl-pine
I've always thought cocaine looked too dualistic, with the two rings & two acetyl branches. 2'-acetoxy-cocaine remedies the branches to a nice triad, but the core of two rings are maintained. Difluoropine is the answer for the rings. I turned the methyl into an ethyl for good measure. However, since due to the difluoropine conformation, I had to reverse the stereochemistry for the optimal isomer, with the 2'-acetoxy branch, it's anybodies guess how it may function. (For instance, it may requiring being on the ring with the other fluorine fluorocarbon, which wouldn't alter my aesthetic specifications)
(edit: it looks a bit like those "BromoDragonFly" molecules, doesn't it? With the tropane as the tail, and the 2'-acetoxy as the head.)
Last edited:
An amateur psychedelic chemist's wet dream
Edit: Another one:
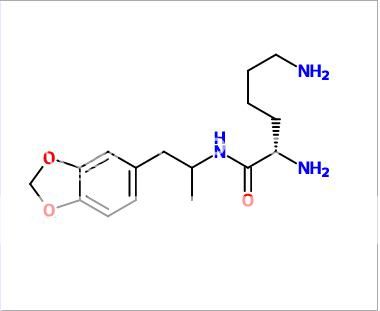
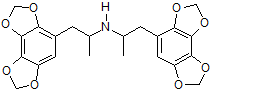
Lismethylenedioxyamphetamine (LisMDA)
Whenever MDA is approved for long term medical use, it'll probably be in this form. (took the idea from Vyvanse)
This one has been opted a few years back among other board (as Lys-MDA) , and has been made.
From what i heard it's orally active but has a delayed come up.
Theoraticly the tripsin-trick seems to work on this compound just as it does on Vyvanse
your Ex.
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
But, it would be interesting to have a variant along the lines of this existing neurosteroid.........

+ Salvinorin A
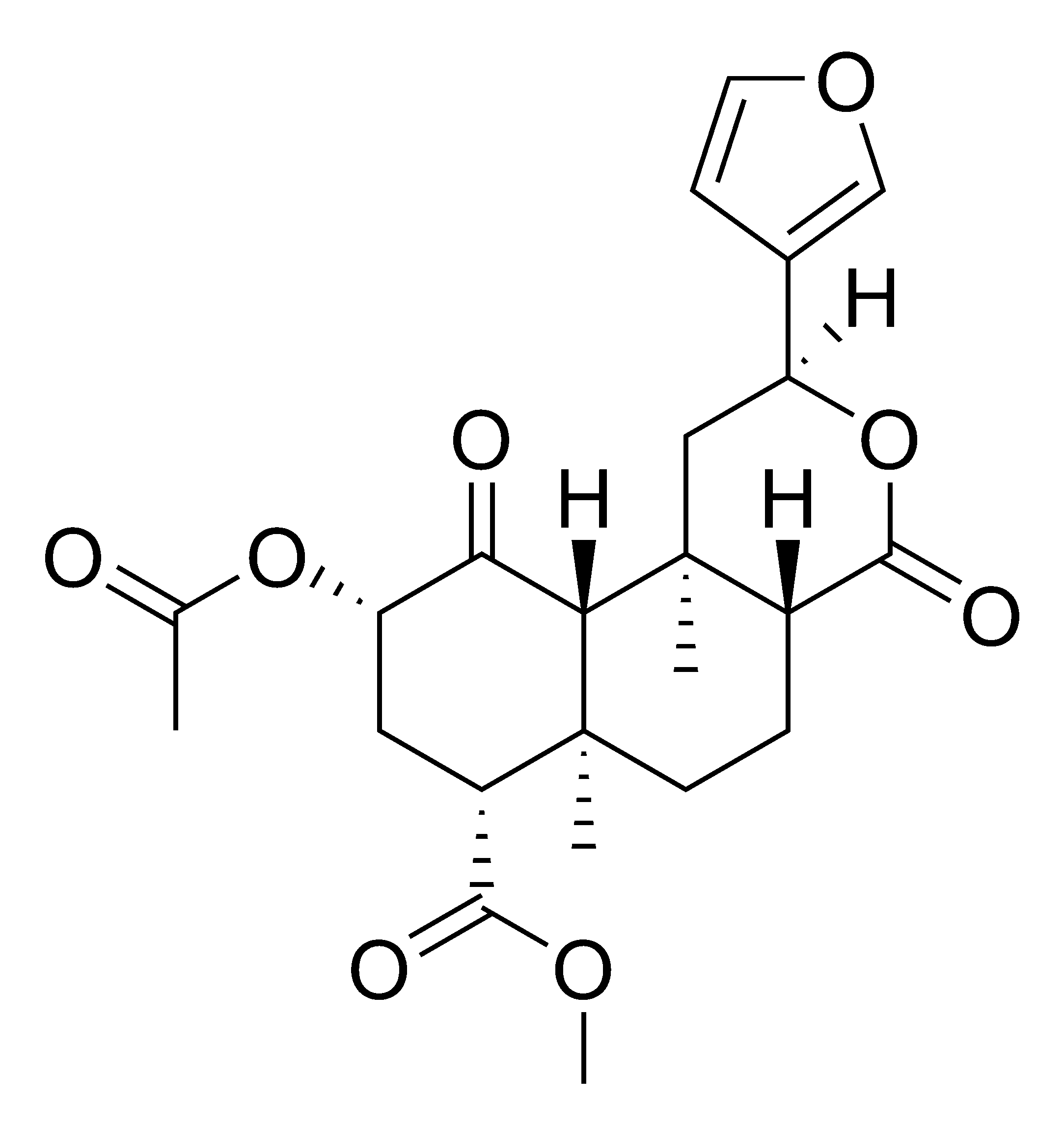
=
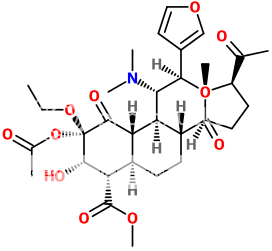
Holy fuck.. it... it overlays...(!)
Salvinorin A is the most centrally active natural molecule known to man. I just made Salvinorin A on steroids (literally).
It looks like an entirely flipped out Swiss army knife though.

Last edited:
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
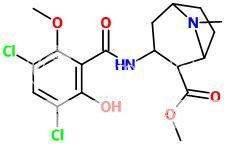
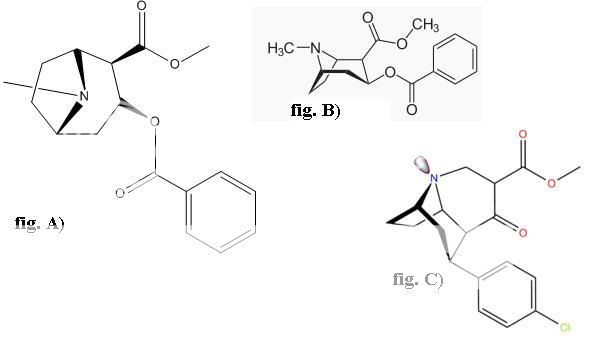
Like this?
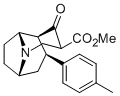
The above doesn't seem like figure C of the below (the ester bridge on the bridged tropane doesn't look correct):
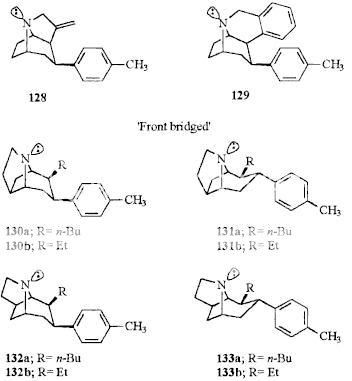
Was it made from one of these? (128?):
I'd still like to see someone's hand at "flattening" (the admittedly already 2D) tropane for that specific "bridged" variant (RTI-242).
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
You guys are awesome! I wish I had the slightest clue what any of this meant...
Yeah... I'll leave now...
Reading and watching what people said for a few years is how I learned anything
Stick around.
FlippingTop
Bluelighter
- Joined
- Sep 7, 2007
- Messages
- 3,211
Fucking awesome thread!
tweaking balls is the best :D
tweaking balls is the best :D
- Status
- Not open for further replies.