Abstract
Background
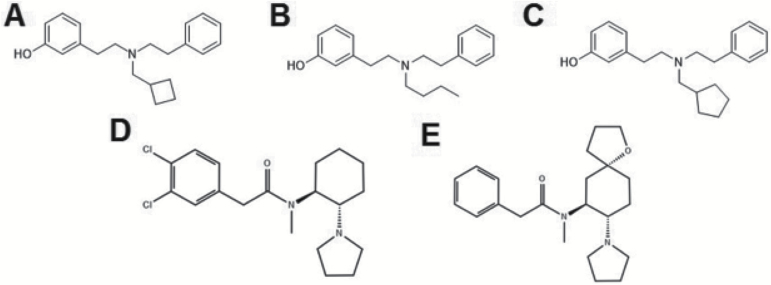
The kappa opioid receptor system has been revealed as a potential pharmacotherapeutic target for the treatment of addictions to substances of abuse. Kappa opioid receptor agonists have been shown to block the rewarding and dopamine-releasing effects of psychostimulants. Recent investigations have profiled the in vivo effects of compounds biased towards G-protein-mediated signaling, with less potent arrestin-mediated signaling. The compounds studied here derive from a series of trialkylamines: N-substituted-N- phenylethyl-N-3-hydroxyphenylethyl-amine, with N-substituents including n-butyl (BPHA), methylcyclobutyl (MCBPHA), and methylcyclopentyl (MCPPHA).
Methods
BPHA, MCBPHA, and MCPPHA were characterized in vitro in a kappa opioid receptor-expressing cell line in binding assays and functional assays. We also tested the compounds in C57BL6 mice, assaying incoordination with rotarod, as well as circulating levels of the neuroendocrine kappa opioid receptor biomarker, prolactin.
Results
BPHA, MCBPHA, and MCPPHA showed full kappa opioid receptor agonism for G-protein coupling compared with the reference compound U69,593. BPHA showed no measurable β-arrestin-2 recruitment, indicating that it is extremely G-protein biased. MCBPHA and MCPPHA, however, showed submaximal efficacy for recruiting β-arrestin-2. Studies in C57BL6 mice reveal that all compounds stimulate release of prolactin, consistent with dependence on G-protein signaling. MCBPHA and MCPPHA result in rotarod incoordination, whereas BPHA does not, consistent with the reported requirement of intact kappa opioid receptor/β-arrestin-2 mediated coupling for kappa opioid receptor agonist-induced rotarod incoordination.
Conclusions
BPHA, MCBPHA, and MCPPHA are thus novel differentially G-protein-biased kappa opioid receptor agonists. They can be used to investigate how signaling pathways mediate kappa opioid receptor effects in vitro and in vivo and to explore the effects of candidate kappa opioid receptor-targeted pharmacotherapeutics.