Synthetic Cathinone Analogues Structurally Related to the Central Stimulant Methylphenidate as Dopamine Reuptake Inhibitors
Corresponding author: Richard A. Glennon (Department of Medicinal Chemistry, School of Pharmacy, Virginia Commonwealth University, Richmond, United States)
ACS Chemical Neuroscience 2020, Volume 10, Issue 9, Pages 4043-4050
Published online August 1st, 2019
https://doi.org/10.1021/acschemneuro.9b00284
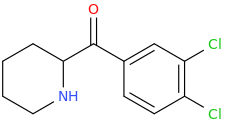
The only cathinone of the series found to be more potent than methylphenidate was 2-(3,4-dichlorobenzoyl)piperidine:
Interestingly, 2-benzylpiperidine was only about 4 times less potent than the corresponding cathinone. If the ring-substitution trend holds for the benzylpiperidines as well, could 2-(3,4-dichlorobenzyl)piperidine be an active DRI at a reasonable dosage?

Corresponding author: Richard A. Glennon (Department of Medicinal Chemistry, School of Pharmacy, Virginia Commonwealth University, Richmond, United States)
ACS Chemical Neuroscience 2020, Volume 10, Issue 9, Pages 4043-4050
Published online August 1st, 2019
https://doi.org/10.1021/acschemneuro.9b00284
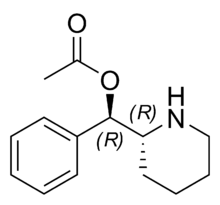
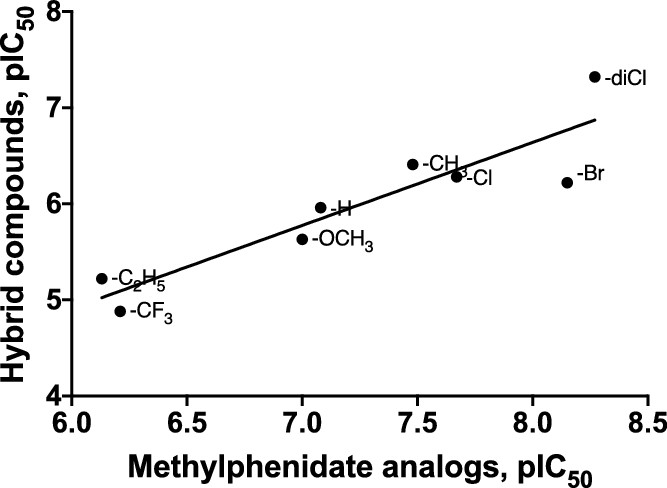
It seems that the cathinone analogues of methylphenidate are significantly less potent DRIs, but the ring-subsitution SAR tracks well:Synthetic cathinones are, primarily, stimulant drugs of abuse that act at monoamine transporters (e.g., the dopamine transporter or DAT) as releasing agents or as reuptake inhibitors. In the past few years, the emergence of >150 new synthetic cathinones has attracted considerable attention from medical and law enforcement communities. threo-Methylphenidate (tMP), used clinically for the treatment of ADHD and narcolepsy, is also a DAT reuptake inhibitor. tMP is somewhat structurally similar to abused cathinone stimulants, and the structure–activity relationships (SAR) of tMP have been well-defined. Hence, available tMP literature might assist in understanding the SAR of synthetic cathinones, about which less is known. In the present study, we synthesized and examined eight 2-benzoylpiperidine analogues (4, 6–12) to determine if tMP SAR might be applicable to cathinone SAR. The benzoylpiperidine analogues were evaluated in a competition assay using live-cell imaging against APP+ in HEK293 cells stably expressing hDAT and in cells coexpressing DAT and voltage-gated Ca2+ channels. All compounds were found to be DAT reuptake inhibitors, and a significant correlation was obtained between the potency of the benzoylpiperidines and tMP binding data (r = 0.91), suggesting that the SAR of tMP analogues might be directly applicable to certain synthetic cathinones as DAT reuptake inhibitors.

The only cathinone of the series found to be more potent than methylphenidate was 2-(3,4-dichlorobenzoyl)piperidine:

Interestingly, 2-benzylpiperidine was only about 4 times less potent than the corresponding cathinone. If the ring-substitution trend holds for the benzylpiperidines as well, could 2-(3,4-dichlorobenzyl)piperidine be an active DRI at a reasonable dosage?

Last edited: