phenethylo J
Bluelight Crew
- Joined
- Jul 23, 2010
- Messages
- 4,932
Black Rose
she'll take you into the darkness
N&PD Moderators: Skorpio | someguyontheinternet



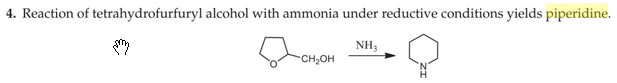

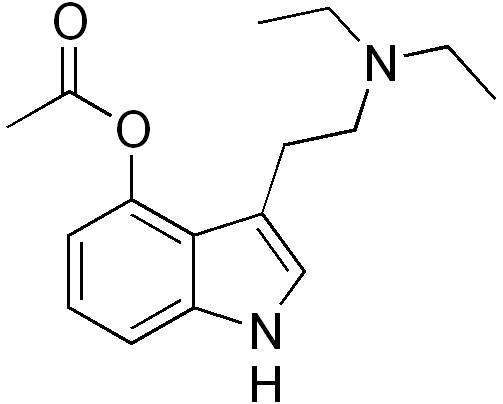
wonder what the mechanism is for this
Looking at the original patent, it's at 175 C under hydrogen over nickel, so my guess is it's just hydrogenolysis of the C-O bond followed by dialkylation of ammonia.
wonder what the mechanism is for this
I would be wary bypassing first-pass metabolism with any kind of unrefined biological mixture.
1. Twelve healthy subjects received 10 mg morphine HCl delivered transdermally from an occlusive reservoir applied to a small area of skin, painlessly de-epithelialised by vacuum suction. On a separate occasion, 10 mg morphine HCl was given as an i.v. infusion over 20 min. 2. Venous blood samples were collected serially for 72 h and assayed for morphine, morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) by h.p.l.c. Pupil size, salivation, and central nervous effects (nausea, fatigue, headache, feeling of heaviness and dysphoria/euphoria) were also measured. 3. After transdermal application morphine was absorbed by a first-order process to produce relatively constant plasma drug concentrations over 11 h. The absolute bioavailability of transdermal morphine was 75% (65-85%; 95% CI). The plasma concentrations of both M6G and M3G were lower after transdermal administration than after i.v. infusion, and a considerable delay (of up to 1 h) was observed before the metabolites were detectable. AUC ratios for M3G and M6G relative to morphine were similar after both modes of administration. 4. Non-analgesic effects were less pronounced at the lower plasma drug and metabolite concentrations observed after transdermal delivery than after the i.v. infusion of morphine. 5. Transdermal administration of morphine warrants investigation as an alternative route of morphine delivery.