-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
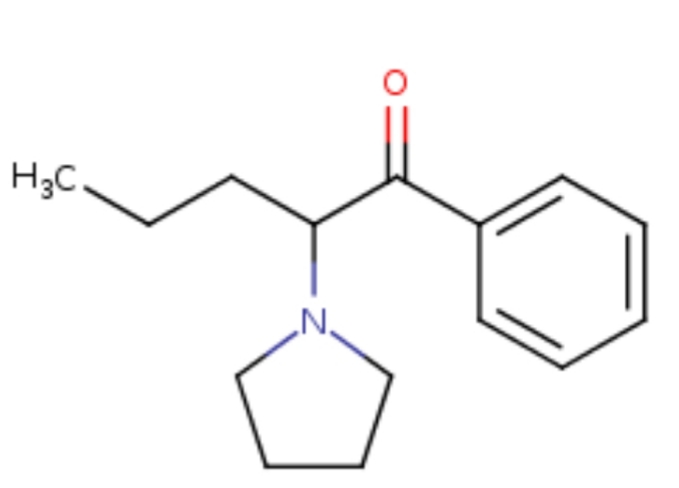
Don't have a name but it's x360 M. If someone can figure out how to add a methyl ether to the 14 then expect that figure to go up a LOT.The TI on it is something like 285. If anyone can figure out how to add a methyl ether to the 14 then it will increase affinity to delta receptors and as well as increasing potency, it will also increase TI. I'm sure people have seen 14-methoxymetopon (x500M) well, check out the TI on that thing. it's truly massive.
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
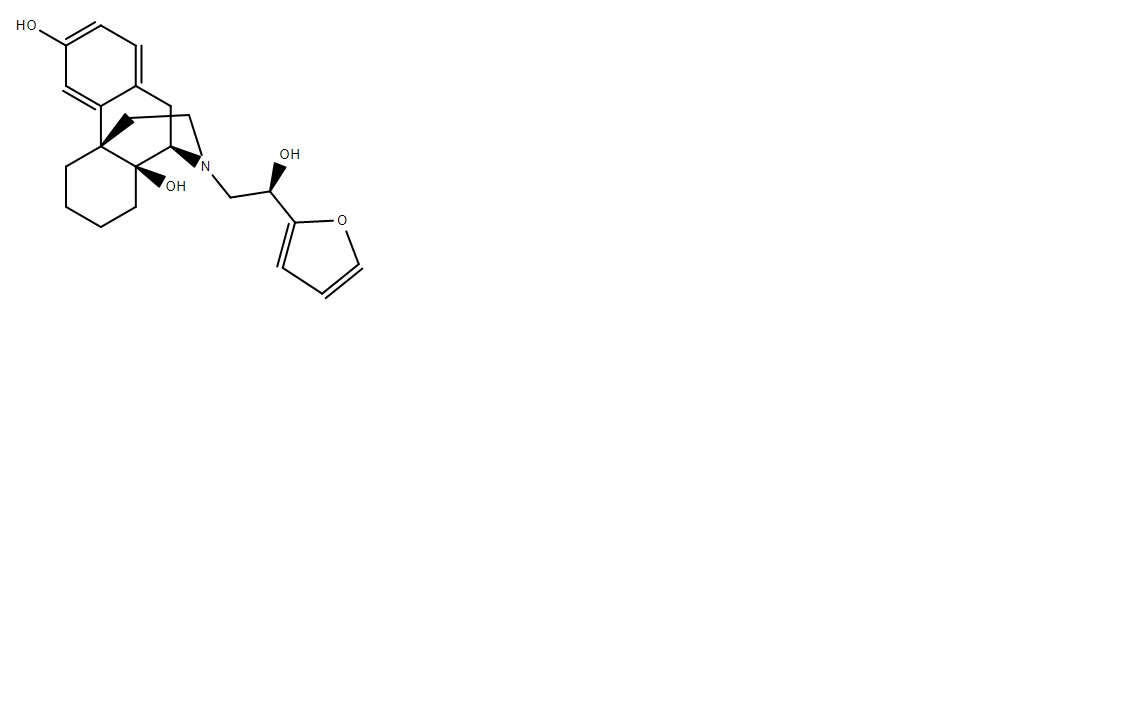
PIPERIPHONE
Ketobemidone's girlfriend, delirious dreams.
EARLY 2000s GERMAN RAVE PILL
Probably the best, usually containing MDMA, AMP, 4MPPP and MDPPP all as a mixture! Those were the good times! (Told to me by a 40 year old raver!) And backed up by papers about MDPPP amd 4MPPP apparitions in Germany in the early 2000s sold along MDMA and AMP in pills.
Last edited:
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work
A. Westbrook, R. van den Bosch, J. I. Määttä, L. Hofmans, D. Papadopetraki, R. Cools, M. J. Frank
Science 20 Mar 2020: Vol. 367, Issue 6484, pp. 1362-1366 DOI: 10.1126/science.aaz5891
Psychostimulants have a place in the therapy of attentional disorders. However, they are also widely used off-label to enhance cognitive performance, and their mechanisms of action remain elusive. Westbrook et al. studied the effects of these drugs and concurrently measured striatal dopamine synthesis capacity in young, healthy participants (see the Perspective by Janes). They administered a placebo, methylphenidate (a dopamine and noradrenaline reuptake blocker), and sulpiride (a selective D2 receptor antagonist) while participants made explicit cost-benefit decisions about whether to engage in cognitive effort. Higher dopamine synthesis capacity in the caudate nucleus was associated with greater willingness to allocate cognitive effort. In addition, methylphenidate and sulpiride increased subjective values and motivation to work specifically for people with low dopamine synthesis capacity. Cognition-enhancing drugs may thus act at the motivational level rather than directly boosting cognition per se.
A. Westbrook, R. van den Bosch, J. I. Määttä, L. Hofmans, D. Papadopetraki, R. Cools, M. J. Frank
Science 20 Mar 2020: Vol. 367, Issue 6484, pp. 1362-1366 DOI: 10.1126/science.aaz5891
Psychostimulants have a place in the therapy of attentional disorders. However, they are also widely used off-label to enhance cognitive performance, and their mechanisms of action remain elusive. Westbrook et al. studied the effects of these drugs and concurrently measured striatal dopamine synthesis capacity in young, healthy participants (see the Perspective by Janes). They administered a placebo, methylphenidate (a dopamine and noradrenaline reuptake blocker), and sulpiride (a selective D2 receptor antagonist) while participants made explicit cost-benefit decisions about whether to engage in cognitive effort. Higher dopamine synthesis capacity in the caudate nucleus was associated with greater willingness to allocate cognitive effort. In addition, methylphenidate and sulpiride increased subjective values and motivation to work specifically for people with low dopamine synthesis capacity. Cognition-enhancing drugs may thus act at the motivational level rather than directly boosting cognition per se.
- Joined
- Aug 16, 2019
- Messages
- 4,688
I am interested to know if this free chemistry script (Java) or other free scripts along the same lines could be added to the Bluelight site? https://opsin.ch.cam.ac.uk
I am not to keen on the use of 'Druglikeness' calculators. I think presenting the results of Lipinski's Rule of 5 alongside the data produced by the above script would be of more value. http://www.niper.gov.in/pi_dev_tools/DruLiToWeb/DruLiTo_index.html
There is even a free version of CHARMM that runs under Linux. That would be a BIG task but the results are just fantastic. https://www.charmm.org/charmm/program/versions/
Even though the site is in Chinese, https://www.drugfuture.com/chemdata/<name> returns the original patents & index papers.
The advantage I see is that people could search for similar chemicals and once a chemical has been added to the system, everyone can see it.
I am prepared to help convert the QSAR data of the ACTIVE drugs in 'Opiates' by R.Lenz et al. It's amazingly thorough but more than 90% of the drugs are inactive. Some drugs not covered by 'Opiates' is covered by 'Opioid Analgesics - Chemistry and Receptors' by Casy and Parfitt. Even then, both books end in the 1980s so there are a lot of much newer compounds.
I am not to keen on the use of 'Druglikeness' calculators. I think presenting the results of Lipinski's Rule of 5 alongside the data produced by the above script would be of more value. http://www.niper.gov.in/pi_dev_tools/DruLiToWeb/DruLiTo_index.html
There is even a free version of CHARMM that runs under Linux. That would be a BIG task but the results are just fantastic. https://www.charmm.org/charmm/program/versions/
Even though the site is in Chinese, https://www.drugfuture.com/chemdata/<name> returns the original patents & index papers.
The advantage I see is that people could search for similar chemicals and once a chemical has been added to the system, everyone can see it.
I am prepared to help convert the QSAR data of the ACTIVE drugs in 'Opiates' by R.Lenz et al. It's amazingly thorough but more than 90% of the drugs are inactive. Some drugs not covered by 'Opiates' is covered by 'Opioid Analgesics - Chemistry and Receptors' by Casy and Parfitt. Even then, both books end in the 1980s so there are a lot of much newer compounds.
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Selective Extraction of Cannabinoid Compounds from Cannabis Seed Using Pressurized Hot Water Extraction
by Yannick Nuapia, Hlanganani Tutu, Luke Chimuka and Ewa Cukrowska
Molecules 2020, 25(6), 1335; https://doi.org/10.3390/molecules25061335
Phytochemicals of Cannabis sativa mainly for the use in the different industries are that of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Pressurized hot water extraction (PHWE) is seen as an efficient, fast, green extraction technique for the removal of polar and semi-polar compounds from plant materials. The PHWE technique was applied to extract cannabinoid compounds from Cannabis sativa seed. Response surface methodology was used to investigate the influence of extraction time (5–60 min), extraction temperature (50–200 °C) and collector vessel temperature (25–200 °C) on the recovery of delta-9-tetrahydrocannabinol (THC), cannabinol (CBN), cannabidiol (CBD), cannabichromene (CBG) and cannabigerol (CBC) from Cannabis sativa seed by PHWE. The identification and semi quantification of cannabinoid compounds were determined using GCXGC-TOFMS. The results obtained from different extractions show that the amount of THC and CBN was drastically decreasing in the liquid extract when the temperature rose from 140 to 160 °C in the extraction cell and the collector′s vessel. The optimal conditions to extract more CBD, CBC, and CBG than THC and CBN were set at 150 °C, 160 °C and 45 min as extraction temperature, the temperature at collector vessel, and the extraction time, respectively. At this condition, the predicted and experimental ratio of THCt (THC + CBN)/CBDt (CBD + CBC+ CBG) was found to be 0.17 and 0.18, respectively. Therefore, PHWE can be seen as an alternative to the classic extraction approach as the efficiency is higher and it is environmentally friendly.
I had no idea you could extract cannabinoids from seeds? And with hot water as a solvent, too? Can you extract hash oil from buds with supercritical steam?
by Yannick Nuapia, Hlanganani Tutu, Luke Chimuka and Ewa Cukrowska
Molecules 2020, 25(6), 1335; https://doi.org/10.3390/molecules25061335
Phytochemicals of Cannabis sativa mainly for the use in the different industries are that of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Pressurized hot water extraction (PHWE) is seen as an efficient, fast, green extraction technique for the removal of polar and semi-polar compounds from plant materials. The PHWE technique was applied to extract cannabinoid compounds from Cannabis sativa seed. Response surface methodology was used to investigate the influence of extraction time (5–60 min), extraction temperature (50–200 °C) and collector vessel temperature (25–200 °C) on the recovery of delta-9-tetrahydrocannabinol (THC), cannabinol (CBN), cannabidiol (CBD), cannabichromene (CBG) and cannabigerol (CBC) from Cannabis sativa seed by PHWE. The identification and semi quantification of cannabinoid compounds were determined using GCXGC-TOFMS. The results obtained from different extractions show that the amount of THC and CBN was drastically decreasing in the liquid extract when the temperature rose from 140 to 160 °C in the extraction cell and the collector′s vessel. The optimal conditions to extract more CBD, CBC, and CBG than THC and CBN were set at 150 °C, 160 °C and 45 min as extraction temperature, the temperature at collector vessel, and the extraction time, respectively. At this condition, the predicted and experimental ratio of THCt (THC + CBN)/CBDt (CBD + CBC+ CBG) was found to be 0.17 and 0.18, respectively. Therefore, PHWE can be seen as an alternative to the classic extraction approach as the efficiency is higher and it is environmentally friendly.
I had no idea you could extract cannabinoids from seeds? And with hot water as a solvent, too? Can you extract hash oil from buds with supercritical steam?
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
F*CK UP
Because fu them, right?
THE PASSENGER
Because he sure ain't driving
"My Heroin has a passenger.. "
ERASER
You won't even see yourself in the mirror
"IT"
The clown of fentantyls.
Was (is?) being sold as CAR-FU, Carfuranylfentanyl.
Last edited:
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Structure–Activity Relationship Study of Psychostimulant Synthetic Cathinones Reveals Nanomolar Antagonist Potency of α-Pyrrolidinohexiophenone at Human Muscarinic M2 Receptors
Yiming Chen, Clinton E. Canal*
ACS Chem. Neurosci. 2020, 11, 6, 960-968 Publication Date:February 19, 2020 https://doi.org/10.1021/acschemneuro.0c00008
Synthetic cathinones (SCs) are designer, psychostimulant drugs of abuse that primarily act on monoamine transporters; little is known about their off-target liability. Abuse of pyrrolidine-containing SCs, such as α-PHP, has been linked to clinical features, including tachycardia and hypertension, and psychiatric events, including delusions and memory impairments—effects mimicking deliriant hallucinogens that are acetylcholine muscarinic receptor (MR) antagonists. α-PHP and nine analogs with modifications in the α-carbon side chain length and/or containing a methylenedioxy moiety were screened for activity at each of the five human MRs. Increasing the length of the α-carbon side chain of 1-phenyl-2-(pyrrolidin-1-yl)ethan-1-one analogs from a methyl (α-PPP) to a propyl (α-PVP) group caused a steep increase in affinity at all MR subtypes, and one extra carbon (α-PHP) further enhanced MR affinity; the presence of a methylenedioxy moiety generally hindered this effect. Highest MR affinity was observed with α-PHP at M2Rs—its M2R affinity (Ki = 251 nM) was 302-fold higher than α-PPP’s. M2R–cAMP inhibition and β-arrestin recruitment assays showed that α-PHP is an M2R antagonist (Kb = 120 and 502 nM, respectively). Additional experiments showed α-PHP is also an antagonist of M1R–inositol phosphate production (Kb = 1.4 μM). Human toxicology studies report blood concentrations of pyrrolidine-containing SCs, including α-PHP, that reach micromolar levels during intoxication, indicating α-PHP’s MR activity might have physiological relevance. As M2Rs and M1Rs are widely expressed in the autonomic and central nervous systems, α-PHP’s anticholinergic activity might be relevant to adverse events associated with α-PHP intoxication.
Yiming Chen, Clinton E. Canal*
ACS Chem. Neurosci. 2020, 11, 6, 960-968 Publication Date:February 19, 2020 https://doi.org/10.1021/acschemneuro.0c00008
Synthetic cathinones (SCs) are designer, psychostimulant drugs of abuse that primarily act on monoamine transporters; little is known about their off-target liability. Abuse of pyrrolidine-containing SCs, such as α-PHP, has been linked to clinical features, including tachycardia and hypertension, and psychiatric events, including delusions and memory impairments—effects mimicking deliriant hallucinogens that are acetylcholine muscarinic receptor (MR) antagonists. α-PHP and nine analogs with modifications in the α-carbon side chain length and/or containing a methylenedioxy moiety were screened for activity at each of the five human MRs. Increasing the length of the α-carbon side chain of 1-phenyl-2-(pyrrolidin-1-yl)ethan-1-one analogs from a methyl (α-PPP) to a propyl (α-PVP) group caused a steep increase in affinity at all MR subtypes, and one extra carbon (α-PHP) further enhanced MR affinity; the presence of a methylenedioxy moiety generally hindered this effect. Highest MR affinity was observed with α-PHP at M2Rs—its M2R affinity (Ki = 251 nM) was 302-fold higher than α-PPP’s. M2R–cAMP inhibition and β-arrestin recruitment assays showed that α-PHP is an M2R antagonist (Kb = 120 and 502 nM, respectively). Additional experiments showed α-PHP is also an antagonist of M1R–inositol phosphate production (Kb = 1.4 μM). Human toxicology studies report blood concentrations of pyrrolidine-containing SCs, including α-PHP, that reach micromolar levels during intoxication, indicating α-PHP’s MR activity might have physiological relevance. As M2Rs and M1Rs are widely expressed in the autonomic and central nervous systems, α-PHP’s anticholinergic activity might be relevant to adverse events associated with α-PHP intoxication.
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Investigation of the Adrenergic and Opioid Binding Affinities, Metabolic Stability, Plasma Protein Binding Properties, and Functional Effects of Selected Indole-Based Kratom Alkaloids
Samuel Obeng, Shyam H. Kamble, Morgan E. Reeves, Luis F. Restrepo, Avi Patel, Mira Behnke, Nelson J.-Y. Chear, Surash Ramanathan, Abhisheak Sharma, Francisco León, Takato Hiranita, Bonnie A. Avery, Lance R. McMahon, Christopher R. McCurdy
Cite this: J. Med. Chem. 2020, 63, 1, 433-439 Publication Date:December 13, 2019 https://doi.org/10.1021/acs.jmedchem.9b01465
Gives Ki values at opioid receptors and adrenergic receptors for several major Kratom alkaloids. The summary:
Corynantheidine half life 6min, Ki 118 nM at mu, 1910 nM at kappa, 41 nM at alpha-1D.
9-Hydroxycorynantheidine, half life 180 min, Ki 105 nM at mu.
Mitragynine half life 20 min, Ki 161 nM at mu, 198 nM at kappa, 1300-9600 nM at the various adrenergic receptors. (1A > 2C > 1B = 2A > 1B > 2B)
7-Hydroxymitragynine half life 170 min, Ki 7 nM at mu, 74 nM at kappa, 236 nM at delta.
Speciociliatine half life 42 min, Ki 54.5 nM at mu, 116 nM at kappa.
Further studies con-ducted to investigate the in vivo functional effects of mitragynine, 7-hydroxymitragynine, and speciociliatine using the hotplate test at 52 ± 0.1 ̊C revealed that 7-hydroxymitragynine and speciociliatine produced maximum response (100 % MPE) with 7-hydroxymitragynine being more potent than speciociliatine and morphine but less potent than fentanyl. Speciociliatine had a similar potency to morphine and mitragynine had the least efficacy among the compounds testedat the highest dose assayed. The antinociceptive effect of 7-hydroxymitragynine was reversed by 0.1 mg/kg naltrexone which suggest that 7-hydroxymitragynine may be acting through the MOP as demonstrated by the binding and in vitro functional as-says. Speciociliatine had antinociceptive effects at 10mg/kg; however, this dose produced lethality in 5/21 rats tested, indicating a narrow therapeutic window. Since the ED50 of speciociliatine to produce antinociception is close to its LD50 value, in this acute antinociception assay (i.e., hotplate test) it was not feasible to evaluate the extent to which the antinociception observed was due to activation of opioid receptors by conducting antagonism tests with opioid sub-type antagonists. In addition, speciociliatine similar to U69593 produced hypothermia but not 7-hydroxymitragynineormitragynine. Collectively, this profile of in vivo activity may indicate that speciociliatine is acting, at least in part, through non-opioids receptors.
Samuel Obeng, Shyam H. Kamble, Morgan E. Reeves, Luis F. Restrepo, Avi Patel, Mira Behnke, Nelson J.-Y. Chear, Surash Ramanathan, Abhisheak Sharma, Francisco León, Takato Hiranita, Bonnie A. Avery, Lance R. McMahon, Christopher R. McCurdy
Cite this: J. Med. Chem. 2020, 63, 1, 433-439 Publication Date:December 13, 2019 https://doi.org/10.1021/acs.jmedchem.9b01465
Gives Ki values at opioid receptors and adrenergic receptors for several major Kratom alkaloids. The summary:
Corynantheidine half life 6min, Ki 118 nM at mu, 1910 nM at kappa, 41 nM at alpha-1D.
9-Hydroxycorynantheidine, half life 180 min, Ki 105 nM at mu.
Mitragynine half life 20 min, Ki 161 nM at mu, 198 nM at kappa, 1300-9600 nM at the various adrenergic receptors. (1A > 2C > 1B = 2A > 1B > 2B)
7-Hydroxymitragynine half life 170 min, Ki 7 nM at mu, 74 nM at kappa, 236 nM at delta.
Speciociliatine half life 42 min, Ki 54.5 nM at mu, 116 nM at kappa.
Further studies con-ducted to investigate the in vivo functional effects of mitragynine, 7-hydroxymitragynine, and speciociliatine using the hotplate test at 52 ± 0.1 ̊C revealed that 7-hydroxymitragynine and speciociliatine produced maximum response (100 % MPE) with 7-hydroxymitragynine being more potent than speciociliatine and morphine but less potent than fentanyl. Speciociliatine had a similar potency to morphine and mitragynine had the least efficacy among the compounds testedat the highest dose assayed. The antinociceptive effect of 7-hydroxymitragynine was reversed by 0.1 mg/kg naltrexone which suggest that 7-hydroxymitragynine may be acting through the MOP as demonstrated by the binding and in vitro functional as-says. Speciociliatine had antinociceptive effects at 10mg/kg; however, this dose produced lethality in 5/21 rats tested, indicating a narrow therapeutic window. Since the ED50 of speciociliatine to produce antinociception is close to its LD50 value, in this acute antinociception assay (i.e., hotplate test) it was not feasible to evaluate the extent to which the antinociception observed was due to activation of opioid receptors by conducting antagonism tests with opioid sub-type antagonists. In addition, speciociliatine similar to U69593 produced hypothermia but not 7-hydroxymitragynineormitragynine. Collectively, this profile of in vivo activity may indicate that speciociliatine is acting, at least in part, through non-opioids receptors.
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
What do you think it would do?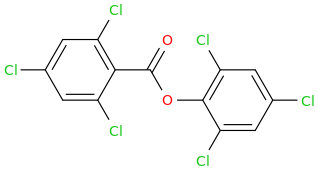
GLOW STICKS
2,4,6-trichlorophenyl-carbonyloxy-2,4,6-trichlorobenzene
See You On The Playa This Coming Labor Day Of Love!!!
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
^ isomeric trichlorobenzoic acids (2,3,6 trichloro) are used as herbicides, acting as auxin analogs.
2,4,6-trichlorophenol is a general biocide (kills everything) that is related to the active ingredient of Dettol antiseptic (chloroxylenol) and also a presumed carcinogen.
2,4,6-trichloroanisole (the methyl ether of 2,4,6, trichlorophenol) is a metabolite of trichlorophenol made in fungus, and has a strongly unpleasant odor. It is found as a flaw in winemaking known as "cork taint".
A closely related compound, the oxalate bis-ester of trichlorophenol, is used as a major component of chemical light sticks, in combination with a fluorescent dye, a weak base, and hydrogen peroxide. That explains the "glow sticks" name.
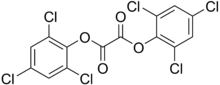
2,4,6-trichlorophenol is a general biocide (kills everything) that is related to the active ingredient of Dettol antiseptic (chloroxylenol) and also a presumed carcinogen.
2,4,6-trichloroanisole (the methyl ether of 2,4,6, trichlorophenol) is a metabolite of trichlorophenol made in fungus, and has a strongly unpleasant odor. It is found as a flaw in winemaking known as "cork taint".
A closely related compound, the oxalate bis-ester of trichlorophenol, is used as a major component of chemical light sticks, in combination with a fluorescent dye, a weak base, and hydrogen peroxide. That explains the "glow sticks" name.

This is the best free editor I know of./www.acdlabs.com/resources/freeware/chemsketch/
If you are ever searching for a commercial chemical, http://www.chemspider.com can be jolly handy.
If you are ever searching for a commercial chemical, http://www.chemspider.com can be jolly handy.
- Status
- Not open for further replies.