-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
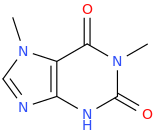
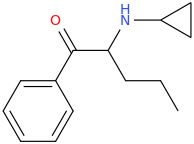
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
- Joined
- Jul 21, 2002
- Messages
- 12,061
Im reminded of 4-benzylpiperidine in #1
I actually included the phrase, "It is most efficacious as a releaser of norepinephrine, with an ec50 of 109/41.4/5246nM for DA/NE/5HT, respectively" in the wiki entry for 4-PMPD.
What memorable addition have you made to a wikipedia article regarding a compound's pharmacology that is extant or in take to this day (in that it remains as written).
I actually included the phrase, "It is most efficacious as a releaser of norepinephrine, with an ec50 of 109/41.4/5246nM for DA/NE/5HT, respectively" in the wiki entry for 4-PMPD.
What memorable addition have you made to a wikipedia article regarding a compound's pharmacology that is extant or in take to this day (in that it remains as written).
Last edited:
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
Disulfiram attenuates morphine or methadone withdrawal syndrome in mice
de Cordé, Annaa; Krząścik, Pawełb; Wolińska, Renataa; Kleczkowska, Patrycjaa; Filip, Małgorzatac; Bujalska-Zadrożny, Magdalena
Behavioural Pharmacology: August 2018 - Volume 29 - Issue 5 - p 393-399
doi: 10.1097/FBP.0000000000000376
Edit: note that disulfiram can be toxic to some people and strain your liver and pancreas, as well as cause neural damage (impaired vision and hearing) due to being converted to carbon disulfide in your body.
de Cordé, Annaa; Krząścik, Pawełb; Wolińska, Renataa; Kleczkowska, Patrycjaa; Filip, Małgorzatac; Bujalska-Zadrożny, Magdalena
Behavioural Pharmacology: August 2018 - Volume 29 - Issue 5 - p 393-399
doi: 10.1097/FBP.0000000000000376
Taking opioids is often accompanied by the development of dependence. Unfortunately, treatment of opioid dependence is difficult, particularly because of codependence – for example, on alcohol or other drugs of abuse. In the presented study, we analyzed the potential influence of disulfiram, a drug used to aid the management of alcoholism, on opioid abstinence syndrome, which occurs as a result of opioid withdrawal. Opioid dependence in mice was induced by subcutaneous administration of either morphine or methadone at a dose of 48 mg/kg for 10 consecutive days. To trigger a withdrawal syndrome, the opioid receptor antagonist, naloxone, was administered at a dose of 1 mg/kg (subcutaneous), and the severity of withdrawal signs was assessed individually. Interruption of chronic treatment with morphine or methadone by naloxone has led to the occurrence of opioid abstinence signs such as jumping, paw tremor, wet-dog shakes, diarrhea, teeth chattering, ptosis, and piloerection. Importantly, pretreatment with disulfiram (25, 50, and 100 mg/kg) reduced the intensity of withdrawal signs induced by naloxone in morphine or methadone-treated mice. These findings show the effectiveness of disulfiram in reducing opioid abstinence signs.
Edit: note that disulfiram can be toxic to some people and strain your liver and pancreas, as well as cause neural damage (impaired vision and hearing) due to being converted to carbon disulfide in your body.
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,448
GEORGIA TECH
N,N-diethyl-13-bromo-lysergamide
BMW
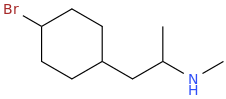
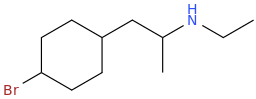
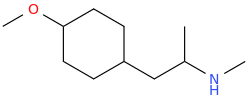
1-(4-methoxycyclohexyl)-2-methylaminopropane
GUCCI
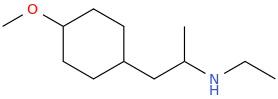
1-(4-methoxycyclohexyl)-2-ethylaminopropane
THE HEALING PURPLE PILL
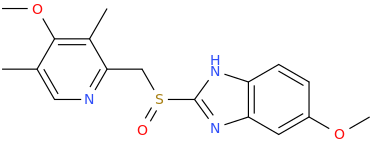
1-(3,5-dimethyl-4-methoxypyridine-2-yl)-1-(6-methoxybenzimidazole-2-yl)sulfinylmethane
ASPIRINA
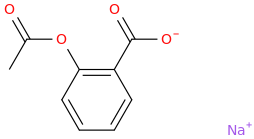
sodium 2-acetoxybenzoate
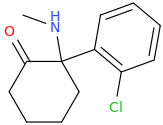
KETAMINE
Special K
2-methylamino-2-(2-chlorophenyl)cyclohexanone
Last edited:
- Joined
- May 11, 2011
- Messages
- 3,377
I've got another one, this time it is a more complete explanation for the mechanism of general anesthetics that takes into account the meyer Overton correlation (the strong correlation between lipophilicity and potency with inhalational anesthetics), but proposes a better mechanism than direct dilution of the lipid membrane.
The mechanism? Disrupting the production of signaling lipids generated by phospholipase D activating TREK1 potassium channels.
Studies on the mechanism of general anesthesia
Mahmud Arif Pavel, View ORCID ProfileE. Nicholas Petersen, View ORCID ProfileHao Wang, View ORCID ProfileRichard A. Lerner, and View ORCID ProfileScott B. Hansen
PNAS first published May 28, 2020 https://doi.org/10.1073/pnas.2004259117
Significance
Anesthetics are used every day in thousands of hospitals to induce loss of consciousness, yet scientists and the doctors who administer these compounds lack a molecular understanding for their action. The chemical properties of anesthetics suggest that they could target the plasma membrane. Here the authors show anesthetics directly target a subset of plasma membrane lipids to activate an ion channel in a two-step mechanism. Applying the mechanism, the authors mutate a fruit fly to be less sensitive to anesthetics and convert a nonanesthetic-sensitive channel into a sensitive one. These findings suggest a membrane-mediated mechanism will be an important consideration for other proteins of which direct binding of anesthetic has yet to explain conserved sensitivity to chemically diverse anesthetics.
Abstract
Inhaled anesthetics are a chemically diverse collection of hydrophobic molecules that robustly activate TWIK-related K+ channels (TREK-1) and reversibly induce loss of consciousness. For 100 y, anesthetics were speculated to target cellular membranes, yet no plausible mechanism emerged to explain a membrane effect on ion channels. Here we show that inhaled anesthetics (chloroform and isoflurane) activate TREK-1 through disruption of phospholipase D2 (PLD2) localization to lipid rafts and subsequent production of signaling lipid phosphatidic acid (PA). Catalytically dead PLD2 robustly blocks anesthetic TREK-1 currents in whole-cell patch-clamp recordings. Localization of PLD2 renders the TRAAK channel sensitive, a channel that is otherwise anesthetic insensitive. General anesthetics, such as chloroform, isoflurane, diethyl ether, xenon, and propofol, disrupt lipid rafts and activate PLD2. In the whole brain of flies, anesthesia disrupts rafts and PLDnull flies resist anesthesia. Our results establish a membrane-mediated target of inhaled anesthesia and suggest PA helps set thresholds of anesthetic sensitivity in vivo.

 www.pnas.org
www.pnas.org
The mechanism? Disrupting the production of signaling lipids generated by phospholipase D activating TREK1 potassium channels.
Studies on the mechanism of general anesthesia
Mahmud Arif Pavel, View ORCID ProfileE. Nicholas Petersen, View ORCID ProfileHao Wang, View ORCID ProfileRichard A. Lerner, and View ORCID ProfileScott B. Hansen
PNAS first published May 28, 2020 https://doi.org/10.1073/pnas.2004259117
Significance
Anesthetics are used every day in thousands of hospitals to induce loss of consciousness, yet scientists and the doctors who administer these compounds lack a molecular understanding for their action. The chemical properties of anesthetics suggest that they could target the plasma membrane. Here the authors show anesthetics directly target a subset of plasma membrane lipids to activate an ion channel in a two-step mechanism. Applying the mechanism, the authors mutate a fruit fly to be less sensitive to anesthetics and convert a nonanesthetic-sensitive channel into a sensitive one. These findings suggest a membrane-mediated mechanism will be an important consideration for other proteins of which direct binding of anesthetic has yet to explain conserved sensitivity to chemically diverse anesthetics.
Abstract
Inhaled anesthetics are a chemically diverse collection of hydrophobic molecules that robustly activate TWIK-related K+ channels (TREK-1) and reversibly induce loss of consciousness. For 100 y, anesthetics were speculated to target cellular membranes, yet no plausible mechanism emerged to explain a membrane effect on ion channels. Here we show that inhaled anesthetics (chloroform and isoflurane) activate TREK-1 through disruption of phospholipase D2 (PLD2) localization to lipid rafts and subsequent production of signaling lipid phosphatidic acid (PA). Catalytically dead PLD2 robustly blocks anesthetic TREK-1 currents in whole-cell patch-clamp recordings. Localization of PLD2 renders the TRAAK channel sensitive, a channel that is otherwise anesthetic insensitive. General anesthetics, such as chloroform, isoflurane, diethyl ether, xenon, and propofol, disrupt lipid rafts and activate PLD2. In the whole brain of flies, anesthesia disrupts rafts and PLDnull flies resist anesthesia. Our results establish a membrane-mediated target of inhaled anesthesia and suggest PA helps set thresholds of anesthetic sensitivity in vivo.

Studies on the mechanism of general anesthesia
Anesthetics are used every day in thousands of hospitals to induce loss of consciousness, yet scientists and the doctors who administer these compounds lack a molecular understanding for their action. The chemical properties of anesthetics suggest that they could target the plasma membrane. Here...
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,448
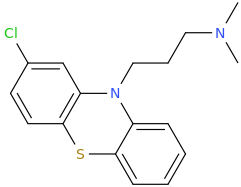
THORAZINE
1-(2-chlorophenothiazin-N-yl)-3-dimethylaminopropane
-antipsychotic (D2 antagonist)
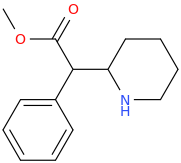
RITALIN
1-phenyl-1-carbomethoxy-(2-piperidinyl)methane
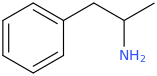
AMPHETAMINE
2-amino-1-phenylpropane
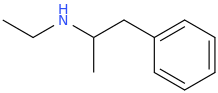
ETHYLAMPHETAMINE
2-ethylamino-1-phenylpropane
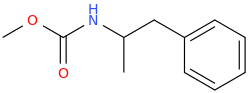
N-carbomethoxy-1-phenyl-2-aminopropane
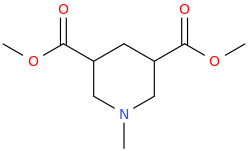
3,5-dicarbomethoxy-N-methylpiperidine
Last edited:
In full disclosure, I spent almost 20 years as a professional games programmer BUT I only ever wrote code in assembly language so I rarely wrote entire games. Others would profile their game and I would rewrite the most time consuming part of the code in optimized assembly language. Sadly, their is little work for people who can ONLY code in such a low-level language (although many of the games I optimized were pretty famous). I mentioned that someone had written a Java IUPAC<-->SMILES<-->Image and I believe that this site uses it:
Now, it IS possible to optimize Java by following a few simple rules. For the truly hardcore, it's possible to write Java AS bytecodes. For those interested, to exit Jazelle (or indeed Java of any flavour):
R0=R1=0
bytecode $ff
But the important thing is that the Java used by this site is free to all. I would LOVE to see an image of the molecule with the IUPAC name underneath it & SMILES below that. I suspect that SMILES itself can be compressed by the simple fact that elements do not appear equally so common elements use short bitstreams while uncommon elements can use longer bitstreams.
Sorry to go on about it but if the site also had an editor, it would allow people to record & produce SMILES & IUPAC naming all within the site. It would avoid images having to be stored on other sites. BL would be a complete resource in 1 place.
Now, it IS possible to optimize Java by following a few simple rules. For the truly hardcore, it's possible to write Java AS bytecodes. For those interested, to exit Jazelle (or indeed Java of any flavour):
R0=R1=0
bytecode $ff
But the important thing is that the Java used by this site is free to all. I would LOVE to see an image of the molecule with the IUPAC name underneath it & SMILES below that. I suspect that SMILES itself can be compressed by the simple fact that elements do not appear equally so common elements use short bitstreams while uncommon elements can use longer bitstreams.
Sorry to go on about it but if the site also had an editor, it would allow people to record & produce SMILES & IUPAC naming all within the site. It would avoid images having to be stored on other sites. BL would be a complete resource in 1 place.
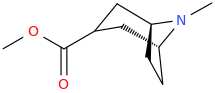
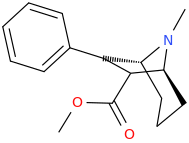
The above compound is around x1239 morphine in animal models. The simpler models that only have a benzene pendant ring have a chiral methyl 1 carbon from benzene & the (S) isomer is about x300M.
I do appreciate that this isn't quite random fluff but it's fascinating how many of Lipinski's rules of 5 & how many of the 'morphine rules' it breaks. A salutary lesson that a piece of semi-science put forward by a senior Pfizer manager saw the entire company reject any an all compounds that broke the rule. It was only published in 1998, just as HTS was a big hit. Now I expect Pfizer to quietly be retesting all of the compounds binned simply for not following this 'rule' or, rather 'rule of thumb'.
S.J.B.
Bluelight Crew
- Joined
- Jan 22, 2011
- Messages
- 6,886
Investigation of the 2,5-Dimethoxy Motif in Phenethylamine Serotonin 2A Receptor Agonists
Corresponding author: Jesper L. Kristensen (Department of Drug Design and Pharmacology, University of Copenhagen, Copenhagen, Denmark)
ACS Chemical Neuroscience 2020, Volume 11, Issue 9, Pages 1238-1244
Published online March 26th, 2020
https://doi.org/10.1021/acschemneuro.0c00129
Corresponding author: Jesper L. Kristensen (Department of Drug Design and Pharmacology, University of Copenhagen, Copenhagen, Denmark)
ACS Chemical Neuroscience 2020, Volume 11, Issue 9, Pages 1238-1244
Published online March 26th, 2020
https://doi.org/10.1021/acschemneuro.0c00129
The 2,5-dimethoxyphenethylamine (2,5-PEA) scaffold is recognized as a motif conferring potent agonist activity at the serotonin 2A receptor (5-HT2AR). The 2,5-dimethoxy motif is present in several classical phenethylamine psychedelics such as 2,4,5- trimethoxyamphetamine (TMA-2), 2,5-dimethoxy-4-methylamphetamine (DOM), 2,5-dimethoxy-4-iodoamphetamine (DOI), 2,5- dimethoxy-4-bromoamphetamine (DOB), 2,5-dimethoxy-4-bromophenethylamine (2C-B), and 2,5-dimethoxy-4-iodophenethylamine (2C-I), and it has previously been suggested that this structural motif is essential for 5-HT2AR activation. In the present study, we present data that challenges this assumption. The 2- and 5-desmethoxy derivatives of 2C-B and DOB were synthesized, and their pharmacological profiles were evaluated in vitro at 5-HT2AR and 5-HT2CR in binding and functional assays and in vivo by assessing their induction of the head-twitch response in mice. Elimination of either the 2- or 5-methoxy group leads to a modest drop in binding affinity and functional potency at 5-HT2AR and 5-HT2CR, which was more pronounced upon removal of the 2-methoxy group. However, this trend was not mirrored in vivo, as removal of either methoxy group resulted in significant reduction in the ability of the compounds to induce the head-twitch response in mice. Thus, the 2,5-dimethoxyphenethylamine motif appears to be important for in vivo potency of phenethylamine 5-HT2AR agonists, but this does not correlate to the relative affinity and potency of the ligands at the recombinant 5-HT2AR.
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
In full disclosure, I spent almost 20 years as a professional games programmer BUT I only ever wrote code in assembly language
Now that's something I admire, much like these which are coded in programming competitions here in Europe:

Demoscene - Wikipedia
I HOPE I am trying to do the best for chemistry. Of course, I don't discuss anything I AM doing on-line but as the patents will show, pyeyzolam (the alcohol mimic) was & is mine. In fact, pynazolam is even more euphoric but I was concerned that it WAS so euphoric. I could see people getting into deep trouble really fast. I suppose a clandestine chemist is supposed to milk a great drug for all it's worth but I always did and have concerned myself with it's safety. Want to know what my criteria is?
'Would I be happy for my own son to take this'
Thus many things were thrown away. OK, my wife ALSO tried everything and even she thought pyeyzolam was just too damned goo.
'Would I be happy for my own son to take this'
Thus many things were thrown away. OK, my wife ALSO tried everything and even she thought pyeyzolam was just too damned goo.
- Status
- Not open for further replies.